|
 |
- Search
AbstractObjectivesTo investigate the validity of newborn hearing screening protocol using automated auditory brainstem response (AABR) with a confirmation method using click auditory brainstem response (ABR) and to evaluate changes in hearing status of infants with confirmed congenital hearing loss.
MethodsNeonates in the well-baby nursery were screened by staged AABR. Subjects whose final AABR result was "refer" were tested by diagnostic click ABR and 226 Hz tympanometry within 3 months of age. Changes in hearing status of subjects with confirmed hearing loss were analyzed by follow-up ABR at 3-6 month intervals.
ResultsOf the 12,193 healthy babies born during this period, 10,879 (89.22%) were screened by AABR. Of 10,879 neonates screened by AABR, 148 (1.36%) were "referred"; of these, 45 subjects showed ABR thresholds over 30 dB nHL in at least one ear. Thirty-four subjects underwent serial follow-up ABR tests, with 11 (32.4%) found to have normal ABR thresholds. Most subjects with mild to moderate hearing loss were found to be normal before 1 year of age, whereas all infants with severe or profound hearing loss were identified as having congenital hearing loss.
ConclusionThe referral rate and the positive predictive value of our protocol were acceptable. We have also found here that substantial temporary hearing loss can be included in the first confirmative diagnosis. Temporary hearing loss of our study on follow-up give emphasis to need of further differentiation using the testing for bone conduction and middle ear status.
Universal newborn hearing screening (UNHS) is now performed worldwide because of the significant harm of unidentified permanent congenital hearing loss. These hearing screening programs have shown benefits for newborns and the Joint Committee on Infant Hearing (JCIH) has renewed up-to-date guidelines for hearing screening using automated auditory brainstem response (AABR) and/or evoked otoacoustic emission (EOAE) (1, 2). These protocols, performed according to the JCIH guidelines, have yielded successful results (3-7).
Among the drawbacks of UNHS is the excessive number of false positives (i.e., normal ears falsely found to have hearing loss) detected using these protocols. About 60-80% of infants referred for further testing were found to be false positives, increasing the rates of medical interventions (3-6). Thus, it is important to reduce the referral rate itself as much as possible. Because UNHS protocols using AABR generally have lower referral rates and better positive predictive values than protocols using EOAE, many institutions utilize AABR protocols (5-7).
Another concern of these screening protocols is the possibility of a change in hearing status after screening protocol. Infants with late onset hearing loss would not be detected by UNHS, suggesting that infants with risk indicators proposed by the JCIH should be rescreened (1). Furthermore, although few follow-up audiology studies after confirmation of congenital hearing loss have been performed, some studies reported children with congenital permanent sensorineural hearing loss undetected with newborn hearing screening (8, 9). Conversely, infants with confirmed hearing loss showing later improvements in hearing were reported (10).
How to confirm congenital hearing loss after newborn screening is another issue. Conventional click air conduction auditory brainstem response (ABR) has been used as a diagnostic method in many previous studies (3-6). Since conventional click ABR may give limited information in differentiation between permanent and temporary, or sensorineural and conductive hearing loss, use of bone conduction ABR in evaluation of failed newborn hearing screening has been reported (8, 11, 12). We have operated a newborn hearing screening program using staged AABR since 2004 with conventional click ABR testing, which is considered to be examined in terms of feasibility with extended follow-up.
We therefore investigated the validity of newborn hearing screening method using AABR, click ABR testing and tympanometry protocol and we also evaluated changes in hearing status of infants with confirmed congenital hearing loss.
We retrospectively reviewed the results of the UNHS process of neonates in the well-baby nursery of Asan Medical Center from March 2004 to December 2009. Our study protocol was approved by the Institutional Review Boards. We included only healthy babies and excluded those treated in or transferred to the neonatal intensive care unit (NICU). Of the 12,193 healthy babies born during this period, 10,879 (89.22%) were screened by AABR (ALGO3; Natus Medical Inc., San Carlo, CA, USA) while asleep and within 24 hours of birth by educated nursing personnel in the nursery. The AABR screener uses a 35 dB nHL alternating polarity click sound to assess the neural response of the auditory nerve. The AABR screener provides a pass-refer result without any need of interpretation. A retest performed immediately after an incomplete result on the initial test was considered part of the initial test. For staged rescreening, infants who failed the previous screening were again tested by AABR on the day prior to discharge.
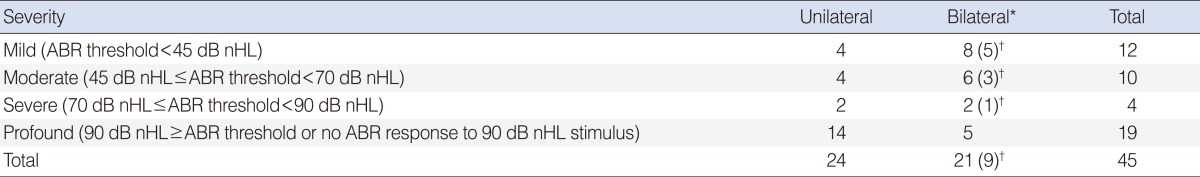
All subjects referred from the hearing screening were recommended to undergo diagnostic testing within 3 months after discharge, as well as to undergo a physical examination by otolaryngologists. A conventional click ABR (Navigator, Biologic Co., Mundelein, IL, USA) with tympanometry (GSI Tympstar, Grason-Stadler, Eden Prairie, MN, USA) at a frequency of 226 Hz were performed by audiologists. For the diagnostic testing, sleep was induced with chlorohydrate syrup (50 mg/kg), electrodes were applied to the forehead and mastoid region, and ABR using click sound was measured. Hearing level was defined as the minimal level resulting in a V wave with filtered click sound. Infants with ABR thresholds above 30 dB nHL in the diagnostic testing were considered to have abnormal hearing, with thresholds less than 45 dB nHL defined as mild hearing loss, thresholds Ōēź45 dB nHL and <70 dB nHL defined as moderate hearing loss, thresholds Ōēź70 dB nHL and <90 dB nHL defined as severe hearing loss, and thresholds of Ōēź90 dB nHL or no ABR response to 90 dB nHL defined as profound hearing loss.
Subsequent to the diagnostic test, a follow-up ABR before 6 months of age was recommended for subjects with abnormal diagnostic ABR results from at least one ear. Babies with bilateral ABR thresholds over 45 dB nHL on follow-up ABR were recommended to use hearing aids for auditory rehabilitation. All subjects with abnormal diagnostic test results, including infants with unilateral hearing loss or bilateral mild hearing loss, were recommended to undergo regular serial follow-up ABR and 226 Hz tympanometry at 3-6 month intervals. Changes in the hearing status of each subject were analyzed with regard to follow-up ABR results. Cochlear implantation was recommended for babies with bilateral profound hearing loss at 1 year of age.
Of the 10,879 neonates initially screened by staged AABR, 148 (1.36%) were "referred". Of these, parents of 117 neonates (79.05%) agreed to diagnostic ABR testing, tympanometry and examination by otolaryngologists (Fig. 1). Forty five infants showed diagnostic ABR thresholds over 30 dB nHL in at least one ear and all except 1 had a type-A tympanogram on the affected side; the remaining subject had a flat type-B tympanogram and middle ear effusion on physical examination. The other 72 of 117 infants showed normal thresholds bilaterally on diagnostic ABR. The positive predictive value of AABR screening was therefore 38.46% (45/117). Of 45 infants with hearing loss, 14 were scored as "refer" in both ears (Fig. 1); of these, 12 had abnormal ABR results in both ears and 2 had abnormal results in only one ear. Twenty two infants showed abnormal ABR results on the "referred" side and normal thresholds on the "pass" side. Nine infants with unilateral AABR "refer" showed bilateral hearing loss (hearing thresholds over 30 dB nHL) during diagnostic ABR and type-A tympanogram. Thus, 24 infants had unilateral and 21 had bilateral abnormal ABR results (Table 1).
Of 45 subjects with hearing loss in diagnostic ABR, 34 infants (75.6%) were assessed by follow-up ABR at least once. The mean length of follow-up was 18.0 months (range, 4 to 48 months) and 21 infants were followed up for over 1 year after birth. We recommended that the hearing status of the 11 infants who did not undergo follow-up ABR at our center be monitored and managed at other centers. Eleven of 34 (32.4%) showed bilateral normal hearing status at follow-up ABR and thresholds of the other 23 (67.6%) remained abnormal throughout their follow-up ABR testing. Two of these infants received ventilation tube insertion at 10 and 11 months of age, respectively, due to persistent middle ear effusion. Of the 9 infants with unilateral AABR "refer" and bilateral hearing loss on diagnostic ABR, 8 showed normal hearing threshold on follow-up ABR. The other one infant was lost to follow-up.
All 6 subjects with mild hearing loss at diagnostic ABR were found to be bilaterally normal on follow-up (Fig. 2). Five of 9 subjects with moderate hearing loss were determined as having normal hearing, whereas the other 4, who showed abnormal thresholds at last follow-up. Bilateral ventilation tube insertion at the age of 6 months was needed in one subject with bilateral moderate hearing loss. Of the 11 subjects with normal hearing on follow-up testing, 10 showed normalization of hearing at 1 year of age; the mean age of normalization in all 11 subjects was 10.3 months (range, 6 to 18 months). The 19 subjects (15 unilateral and 4 bilateral hearing loss) with severe or profound hearing loss at diagnostic ABR remained having hearing loss on follow-up. Of these 19, 2 underwent ventilation tube insertion and 5 were lost to follow-up before 1 year of age. The other 12 subjects continued to show abnormal follow-up ABR results after 1 year of age.
Of the 23 subjects with hearing loss on follow-up ABR, 3 (13.0%) showed changes in severity of hearing loss at last follow-up ABR. One infant with severe unilateral hearing loss and 1 with moderate bilateral hearing loss improved to moderate unilateral and mild bilateral hearing loss, respectively. One infant with moderate unilateral hearing loss progressed to severe hearing loss during follow-up, but he also had middle ear effusion in the affected ear. We performed cochlear implantations for 3 babies with bilateral profound hearing loss and prescribed hearing aids for 3 babies with bilateral moderate to severe hearing loss in this study cohort.
We screened 90% of healthy newborns for UNHS over a period of about 6 years, with nearly 80% of referred neonates followed up for confirmation. Although our protocol did not reach a capture rate of 95% or a follow-up rate of 90%, the quality indicators of the JCIH in 2007, we consider our rates remarkable for Korea, a country where newborn hearing screening is not fully supported by the national health insurance service (1). The referral rate was 1.36%, with 45 true positives and 72 false positives identified through the screening protocol. Thus, of over 10,000 infants screened, 0.41% was true positives of screening results, and the positive predictive value of our protocol was 38.46%, both of which are acceptable and comparable with other studies (3-7, 10). Thirteen infants (0.12%) had greater than moderate hearing loss bilaterally, requiring hearing rehabilitation; this incidence of congenital hearing loss was similar to those shown previously (3-7, 10).
Follow-up ABR was performed to assess the initial diagnostic ABR result and the accuracy of the first diagnosis. Of 34 infants followed up serially, about one-third had normal hearing status during follow-up ABR, indicating that the cases, initially regarded as having hearing loss judged by diagnostic ABR, were actually normal in hearing. Moreover, of the subjects with hearing loss on diagnostic ABR, some infants showed changes in severity of hearing loss on follow-up ABR. Such normalization of hearing or changes in severity of hearing loss was noticeable in subjects with mild or moderate hearing loss. Of the 6 subjects with mild hearing loss on diagnostic ABR, none had hearing loss on follow-up and only 4 of 9 subjects with moderate hearing loss on diagnostic ABR were found to have hearing loss finally. In contrast, subjects with severe or profound hearing loss on diagnostic ABR showed relatively robust results on follow-up.
One of the possible causes of the change of hearing level may be related to the middle ear inflammation. Considering the incidence of middle ear effusions in infancy is estimated to be as high 61%, subjects with middle ear effusion can be involved in our study population (13). Actually, middle ear opacity on high resolution computed tomography (HRCT) has been reported in about 40% of infants with bilateral hearing loss of more than 50 dB identified by newborn hearing screening (10). Another possible explanation of hearing improvement can be delayed auditory pathway maturation. Talero-Gutierrez et al. (14) reported spontaneous recovery of hearing threshold in children around 1 year of age.
The key point of distinguishing temporary hearing loss due to middle ear effusion from permanent congenital hearing loss is complete evaluation for middle ear pathology. For this purpose, we used otoscopic examination and 226 Hz tympanometry which have substantial limitation for clear conclusion. Physical examination of the tympanic membrane in infants may be inaccurate due to technical difficulties, such as narrow ear canals, the presence of cerumen, and the lack of cooperation, and may also contribute to a misdiagnosis of middle ear effusion (15, 16). Furthermore, a study of infants less than 7 months old demonstrated that the result of tympanometry is often normal in the presence of middle ear effusion (17). In addition, standard 226 Hz tympanometry may not be appropriate for hearing screening in infants less than 6 months of age because of its incorrect and unreliable results and the JCIH has recommended that these infants be screened by 1,000 Hz tympanometry (1, 18, 19). If temporary middle ear effusion resolved during follow-up, follow-up ABR would show normal results. Because we performed only air conduction click ABR testing and 226 Hz tympanometry throughout follow-up, it might not be able to detect middle ear effusion in considerable number of subjects. Thus, undetected middle ear effusion at the time of diagnostic ABR may explain our temporary hearing loss findings.
In addition, we identified 9 ears that passed initial AABR screening and proved as having hearing loss on diagnostic ABR. Since none of these infants was suspected of having middle ear effusion at the time of diagnostic ABR, they could be regarded as having undetected congenital hearing loss which could threaten the feasibility of newborn hearing screening protocols. However, because all the patients except one showed normal hearing during follow-up and one of these patients was follow-up loss, there was no false negative case found in our series.
Change of hearing thresholds on follow-up ABR testing draws attention to the need of clear discrimination of type of hearing loss at the time of diagnostic testing. Many previous studies have concentrated on methods and results of the screening procedure and provided less information about the confirmation method and how to they clearly exclude the temporary conductive hearing loss (3-6). As seen in this study, however, conventional click ABR and 226 Hz tympanometry are not enough to evaluate all possible hearing conditions. Some recent studies implemented bone conduction click or tone burst ABR with/without 1,000 Hz tympanometry in evaluation of failed newborn hearing screening and it can bring proper early diagnosis by distinguishing amongst permanent sensorineural hearing loss, permanent conductive hearing loss, and temporary conductive hearing loss (8, 11, 12).
JCIH guidelines have emphasized that hearing interventions be performed before 6 months of age, and there is increasing interest in the benefits of auditory rehabilitation for unilateral or mild bilateral hearing loss (1, 20, 21). Excessive false positive cases due to middle ear effusions can result in additional unnecessary medical interventions, put an emotional burden of parents and lower the specificity and positive predictive value of the screening method. Our results indicate that hearing status at the time of diagnostic testing is uncertain with conventional click ABR and 226 Hz tympanometry, especially in cases of mild to moderate hearing loss and raise the need for implementation of more tools for bone conduction and middle ear evaluation to clarify whether temporary hearing loss of our study is temporary conductive or sensorineural hearing loss.
Taken together, the referral rate and the positive predictive value of our protocol were acceptable. We have also found here that substantial temporary hearing loss can be included in the first confirmative diagnosis. Temporary hearing loss of our study on follow-up give emphasis to need of further differentiation using the testing for bone conduction and middle ear status. Complete evaluation of type and severity of hearing loss is important to avoid excessive burden from follow-up and to provide proper early intervention for permanent hearing loss.
References1. American Academy of Pediatrics. Joint Committee on Infant Hearing. Year 2007 position statement: principles and guidelines for early hearing detection and intervention programs. Pediatrics. 2007 10;120(4):898-921. PMID: 17908777.
2. Joint Committee on Infant Hearing. American Academy of Audiology. American Academy of Pediatrics. American Speech-Language-Hearing Association. Directors of Speech and Hearing Programs in State Health and Welfare Agencies. Year 2000 position statement: principles and guidelines for early hearing detection and intervention programs. Joint Committee on Infant Hearing, American Academy of Audiology, American Academy of Pediatrics, American Speech-Language-Hearing Association, and Directors of Speech and Hearing Programs in State Health and Welfare Agencies. Pediatrics. 2000 10;106(4):798-817. PMID: 11015525.
3. Korres S, Nikolopoulos TP, Peraki EE, Tsiakou M, Karakitsou M, Apostolopoulos N, et al. Outcomes and efficacy of newborn hearing screening: strengths and weaknesses (success or failure?). Laryngoscope. 2008 7;118(7):1253-1256. PMID: 18401271.
4. Iwasaki S, Hayashi Y, Seki A, Nagura M, Hashimoto Y, Oshima G, et al. A model of two-stage newborn hearing screening with automated auditory brainstem response. Int J Pediatr Otorhinolaryngol. 2003 10;67(10):1099-1104. PMID: 14550964.
5. Benito-Orejas JI, Ramirez B, Morais D, Almaraz A, Fernandez-Calvo JL. Comparison of two-step transient evoked otoacoustic emissions (TEOAE) and automated auditory brainstem response (AABR) for universal newborn hearing screening programs. Int J Pediatr Otorhinolaryngol. 2008 8;72(8):1193-1201. PMID: 18550180.
6. Lin HC, Shu MT, Lee KS, Ho GM, Fu TY, Bruna S, et al. Comparison of hearing screening programs between one step with transient evoked otoacoustic emissions (TEOAE) and two steps with TEOAE and automated auditory brainstem response. Laryngoscope. 2005 11;115(11):1957-1962. PMID: 16319605.
7. Vohr BR, Oh W, Stewart EJ, Bentkover JD, Gabbard S, Lemons J, et al. Comparison of costs and referral rates of 3 universal newborn hearing screening protocols. J Pediatr. 2001 8;139(2):238-244. PMID: 11487750.
8. Johnson JL, White KR, Widen JE, Gravel JS, James M, Kennalley T, et al. A multicenter evaluation of how many infants with permanent hearing loss pass a two-stage otoacoustic emissions/automated auditory brainstem response newborn hearing screening protocol. Pediatrics. 2005 9;116(3):663-672. PMID: 16140706.
9. Wada T, Kubo T, Aiba T, Yamane H. Further examination of infants referred from newborn hearing screening. Acta Otolaryngol Suppl. 2004 10;(554):17-25. PMID: 15513505.
10. Adachi N, Ito K, Sakata H, Yamasoba T. Etiology and one-year follow-up results of hearing loss identified by screening of newborn hearing in Japan. Otolaryngol Head Neck Surg. 2010 7;143(1):97-100. PMID: 20620626.
11. Holster IL, Hoeve LJ, Wieringa MH, Willis-Lorrier RM, de Gier HH. Evaluation of hearing loss after failed neonatal hearing screening. J Pediatr. 2009 11;155(5):646-650. PMID: 19616786.
12. Shoup AG, Owen KE, Jackson G, Laptook A. The Parkland Memorial Hospital experience in ensuring compliance with universal newborn hearing screening follow-up. J Pediatr. 2005 1;146(1):66-72. PMID: 15644825.
13. Casselbrant ML, Brostoff LM, Cantekin EI, Flaherty MR, Doyle WJ, Bluestone CD, et al. Otitis media with effusion in preschool children. Laryngoscope. 1985 4;95(4):428-436. PMID: 4039020.
14. Talero-Gutierrez C, Carvajalino-Monje I, Samper BS, Ibanez-Pinilla M. Delayed auditory pathway maturation in the differential diagnosis of hypoacusis in young children. Int J Pediatr Otorhinolaryngol. 2008 4;72(4):519-527. PMID: 18243343.
15. Ho V, Daly KA, Hunter LL, Davey C. Otoacoustic emissions and tympanometry screening among 0-5 year olds. Laryngoscope. 2002 3;112(3):513-519. PMID: 12148864.
16. Engel J, Anteunis L, Chenault M, Marres E. Otoscopic findings in relation to tympanometry during infancy. Eur Arch Otorhinolaryngol. 2000 7;257(7):366-371. PMID: 11052246.
17. Paradise JL, Smith CG, Bluestone CD. Tympanometric detection of middle ear effusion in infants and young children. Pediatrics. 1976 8;58(2):198-210. PMID: 951134.
18. Shahnaz N, Miranda T, Polka L. Multifrequency tympanometry in neonatal intensive care unit and well babies. J Am Acad Audiol. 2008 5;19(5):392-418. PMID: 19253812.
19. Keefe DH, Gorga MP, Neely ST, Zhao F, Vohr BR. Ear-canal acoustic admittance and reflectance measurements in human neonates: II. predictions of middle-ear in dysfunction and sensorineural hearing loss. J Acoust Soc Am. 2003 1;113(1):407-422. PMID: 12558278.
20. Fitzpatrick EM, Durieux-Smith A, Whittingham J. Clinical practice for children with mild bilateral and unilateral hearing loss. Ear Hear. 2010 6;31(3):392-400. PMID: 20054278.
21. Tharpe AM. Unilateral and mild bilateral hearing loss in children: past and current perspectives. Trends Amplif. 2008 3;12(1):7-15. PMID: 18270174.
Fig.┬Ā1Flowchart and outcomes of newborn hearing screening with staged automated auditory brainstem response (AABR). *Number of infants with true positive ear in at least 1 ear. ŌĆĀNumber of infants with bilateral false positive ears. ABR, auditory brainstem response; HL, hearing loss. 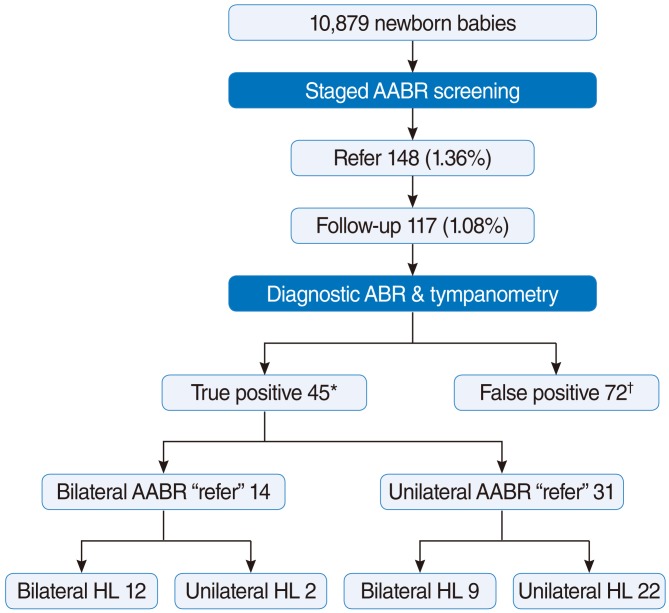
Fig.┬Ā2Relationship between the severity of diagnostic auditory brainstem response (ABR) and the results of follow-up ABR. Mild, ABR threshold<45 dB nHL; Moderate, 45 dB nHLŌēżABR threshold<70 dB nHL; Severe, 70 dB nHLŌēżABR threshold<90 dB nHL; Profound, ABR thresholdŌēź90 dB nHL or no ABR response to 90 dB nHL stimulus. 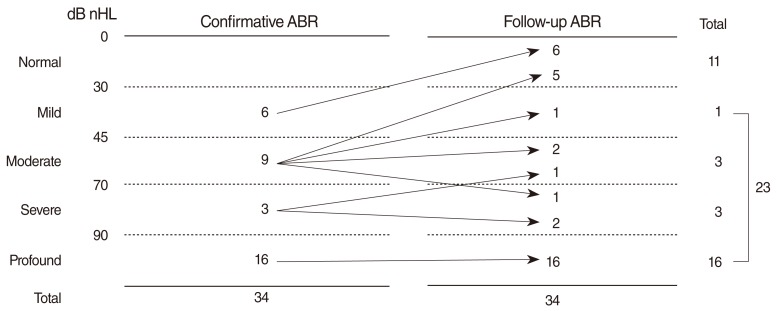
|
|
||||||||||||||||||||||||||||||||||||||