INTRODUCTION
Otitis media with effusion (OME) is one of the most common diseases in infants and children, with more than 90% of children experiencing OME before reaching school age (1, 2). About 2.2 million diagnosed episodes of OME occurs annually in the United States, and more than one million operations are performed (3). As a result, many children suffer from OME, and the medical cost is very high. Nevertheless, fundamental treatment and prevention of OME have not yet been completely determined. If OME is not adequately treated, then resultant hearing difficulty can cause delayed language development, intellectual disorders, and communication problems (2, 3).
The etiologies of OME are very diverse and include viral and bacterial infection, Eustachian tube dysfunction, and excessive exudate production due to allergy and chronic inflammation (4, 5). Therefore, management of OME varies depending on the patient and the clinician, and such management may be pursued through observation, antibiotic therapy, or ventilation tube (VT) insertion (3, 4, 6, 7). There is considerable debate among physicians concerning the treatment principles of OME: are antibiotics or antihistamines necessary? How long should antibiotics be used? When is VT insertion needed?
Recently, new evidence-based recommendations have been introduced for the diagnosis and management of OME in children (8, 9). These are presently the best uidelines, based on the analysis of results selected from many studies. The updated 2004 guidelines offer several recommendations, including atchful waiting for three months, and issue recommendations related to the avoidance of antihistamines, decongestants, antimicrobials, and corticosteroids (9). In addition, the subcommittee has stated that the guidelines are designed to assist primary care physicians and has emphasized the significance of documentation related to laterality, duration of effusion, and presence and severity of associated symptoms, in the interest of facilitating communication between referring primary care clinicians and specialists (9). However, there are some difficulties in following the general guidelines in the tertiary hospital setting, because referred children have often been treated with medications already, and they may also have allergies and sinusitis (10-12). Furthermore it is often hard to make decisions related to further management, including VT insertion, because of imprecise information and documentation (12). In the present study, we tried to apply the guidelines to referred children with OME and to evaluate the efficiency of antibiotics and antihistamines in the management of OME in a tertiary hospital setting.
MATERIALS AND METHODS
One hundred children with OME diagnosed by pneumatic otoscopy, tympanography, and pure tone audiography in the tertiary Ajou University Hospital (Suwon, Korea), between June 2003 and March 2005, were enrolled in this consecutive, randomized study. The Institutional Review Board of the Ajou University School of Medicine (Suwon, Korea) approved the study protocol. The diagnosis of OME was limited to patients showing B or C type on tympanometry and exhibiting hearing loss greater than 25 dB on pure tone audiometry. Patients with acute otitis media (AOM) and fever or otalgia were excluded from the study. We also excluded children with cleft palates, developmental difficulties, contraindications to medications, and follow-up loss. All patients were diagnosed by one experienced otologist. Sixteen of 100 children were lost to follow-up, so 84 children were eventually included in the analysis. There were 57 boys and 27 girls, and the average age was 69.0 months (range, 5 months-12 yr).
These patients were randomized to receive five different kinds of treatment for two weeks, regardless of antibiotic/antihistamine/steroid medication history. After obtaining consent from parents or guardians, we consecutively and randomly prescribed an antibiotic, amoxicillin-clavulanate syrup (1 cc/kg, Augmex Duo syrup®, Korea United Parma. INC., Seoul, Korea), in Group I (n=16); a combination of an antibiotic and a steroid, prednisolone (1 mg/kg, Solondo®, Yuhan corp., Seoul, Korea), in Group II (n=18); a combination of an antibiotic and an antihistamine, ebastine (0.2 cc/kg, Ebastel®, Boryung INC., Seoul, Korea), in Group III (n=15); a combination of an antibiotic, a steroid, and an antihistamine in Group IV (n=17); and a mucolytic ivy leaf extract (0.5 cc/kg, Prospan syrup®, Ahn-Gook Pharm corp., Seoul, Korea) alone in Group V (n=17). We used the mucolytics as a control.
In the present study, amoxicillin-clavulanate was used as the basic antibiotic medication for patients with OME visiting the tertiary hospital, while considering previous history of antibiotic use and possibility of organism resistance to first-line antibiotics. Ampicillin and amoxicillin are the first-line medications usually used for treatment of OME in local primary clinics in Korea. Amoxicillin-clavulanate is also frequently used in local clinics for treating patients with long histories of OME, because it is effective in treating ╬▓-lactamase producing bacteria.
The children were followed up every 2 weeks, tympanometry and pure tone audiometry were obtained, and decisions were made regarding cure, observation, or operation, based on the usual recommendations. VT insertion was performed to patients with hearing loss greater than 40 dB, in patients with bilateral OME for greater than three months, and in patients with unilateral OME for greater than six months. We observed patients whose hearing loss was less than 40 dB for three months if bilateral OME was present and for six months if unilateral OME was present. The values are presented as means┬▒standard deviations (SD). Statistical analyses were performed using a Pearson chi square test. P<0.05 were considered statistically significant.
RESULTS
Clinical characteristics of children with OME visiting a tertiary hospital (Table 1)
The mean sustained period (past history) of OME after the first attack was 12.9┬▒12.3 months, and the mean duration of recent OME occurring just before visiting our hospital was 10.5┬▒14.8 weeks. The mean duration of medical therapy for OME was 5.1┬▒6.2 weeks, and the mean frequency of upper respiratory infections (URI) was 2.9┬▒1.6 times per six months. There were no significant differences among the five groups with regard to duration of recent OME, medication history, or frequency of URIs (P>0.05).
Sixty-eight patients (81.0%) had bilateral OME, and 16 patients (19.0%) had unilateral OME. At the time of referral to our hospital, the air conduction threshold and the air-bone gap were 26.1┬▒11.3 dB and 22.1┬▒13.6 dB on the right side and 26.4┬▒11.0 dB and 23.8┬▒12.1 dB on the left side, respectively.
Thirty-four (40.5%) patients exhibited two or more allergic symptoms, including itching, sneezing, nasal obstruction, or watery rhinorrhea. We found no allergic symptoms in 46 (54.8%) patients. Four children (9.5%) had insufficient information concerning allergies. Forty children (47.6%) who were suspected of having allergies underwent allergic skin tests. Seventeen (42.5%) of these children exhibited positive responses, and 23 (57.5%) exhibited negative responses.
Results of the management of OME
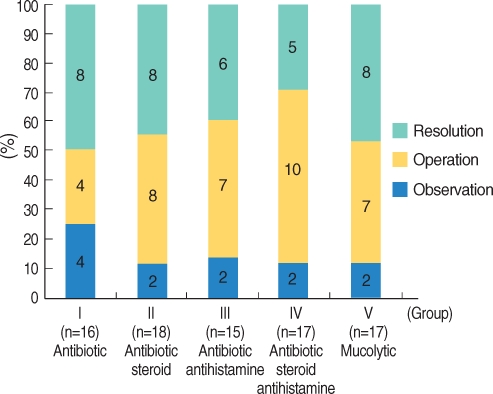
Thirty-six (42.9%) of 84 children resolved over a mean period of 6.9 weeks after initiation of treatment. Thirty-six children (42.9%) were treated with VT insertion, and 12 children (14.3%) were continuously observed (Fig. 1). Groups I, II, III, IV, and V showed resolution rates of 50.0%, 44.4%, 40.0%, 29.4%, and 47.1%, respectively. The OME resolution rates were no different among the groups (P>0.05). The ventilation tube insertion rates were 25.0% in group I, 44.4% in group II, 46.7% in group III, 58.8% in group IV, and 41.2% in group V (Fig. 2).
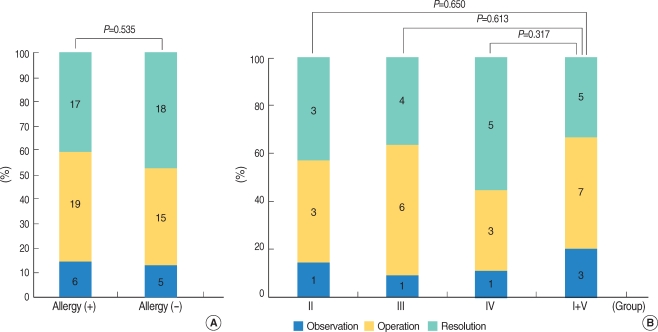
We analyzed whether allergic symptoms or positive skin tests affected the prognosis of OME. However, we found no significant differences in the resolution rates for the allergic group (n=42) and the non-allergic group (n=38) (Fig. 3A). There were no differences in the resolution rates for the groups (n=42) with allergic symptoms or positive skin test responses, when steroids (group II), antihistamines (group III), steroids and antihistamines (group IV), or other medications (group I and V) were used (P>0.05) (Fig. 3B).
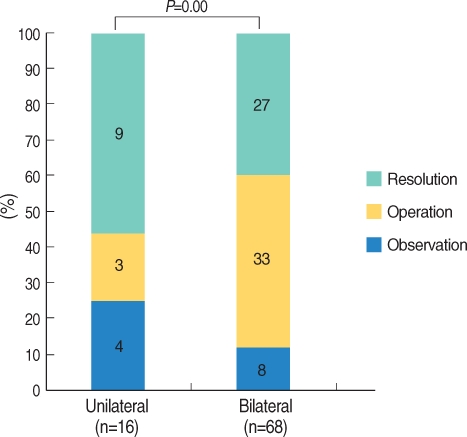
Regarding the management of OME according to laterality, 56.3% of children with unilateral OME (n=16) experienced resolution, a figure statistically higher than the 39.7% resolution rate seen in bilateral OME (n=68) (P=0.000) (Fig. 4).
DISCUSSION
OME is defined as the presence of fluid in the middle ear without signs or symptoms of acute ear infection. It can be detected on routine screening tests, and it can be diagnosed using AOM. There is considerable debate concerning the treatment of OME because patients generally do not have severe symptoms, and diverse treatment trials show conflicting results. Therefore, practical guidelines for aiding the diagnosis and treatment of OME children, aged 2 months to 12 yr, have been developed by experts in the fields of primary care, otolaryngology, infectious disease, epidemiology, hearing, speech and language, and advanced practice nursing (8, 9).
Specifically, the 2004 guidelines offer 11 evidence-based statements and divide treatment into four groups based on clinical presentation: strong recommendation, recommendation, option, and no recommendation (9). According to these guideline, VT insertion is recommended in children with OME after three to six months of watchful waiting. This recommendation is based on the notion that OME often resolves spontaneously. Approximately 90% of cases resolve by three months (13), and only 5-10% persist for more than one year (1, 14). In the present study, we tried to apply watchful waiting as long as possible, following the guidelines. However, the total resolution rate after two weeks of medication and watchful waiting was only 42.9%. This figure was much lower than we expected. Actually, we could not avoid performing VT insertion in 36 patients (42.9%) because of prolonged or severe hearing loss or speech problems. These results indicate that the natural resolution rate of OME in children who visit a tertiary hospital is very low and that some patients may be refractory to early treatments such as short-term medical therapy in the tertiary hospital setting in South Korea.
Consequently, the following guideline amendments seem to be appropriate. Clinicians should look for children with OME who are at risk for speech, language, or learning problems and should promptly evaluate hearing, speech, language, and need for intervention in these patients (9). We believe prompt, precise evaluation can help clinicians determine the appropriate approach to referred patients, thus avoiding unnecessary watchful waiting. It should be mentioned that the guidelines are not intended as a sole source of guidance in evaluating children with OME (9).
Therefore, it is very important to have precise information and documentation from primary care clinicians, as the guidelines already emphasize. Primary care doctors should provide doctors in the tertiary hospitals with information concerning effusion duration, specific reasons for referral, and additional relevant information, such as history of AOM and developmental status of the child. However, in many patients with OME, the information is not delivered correctly or in detail, but is given only by the patient's parent or caregiver. Therefore, specialists treating OME in the tertiary hospital need to develop a form and referral system connecting them with the primary care clinicians.
Evidence-based recommendations indicate that antihistamines and decongestants are generally ineffective for the treatment of OME and are therefore not recommended. Furthermore, antimicrobials and corticosteroids do not have long-term efficacy and are not recommended for routine management (8, 9). However, we believe these recommendations are more applicable in the primary care setting, not in referred patients with severe or prolonged symptoms. In fact, most referred children have already received many medications, including antibiotics, and many of them have a history of allergic rhinitis and sinusitis. Therefore, we prospectively designed and applied five different protocols made up of different combinations of antibiotics, antihistamines, and corticosteroids, in the interest of identifying their effectiveness. There were no statistically significant differences among the five groups (P>0.05). In addition, children with allergic symptoms or signs in the present study showed no significant responses to antihistamines or corticosteroids (P>0.05). These findings are in agreement with the 2004 clinical practice guidelines for the treatment of OME (6, 9, 15, 16).
Concerning the management of OME according to laterality, children with unilateral OME in the present study had better prognosis than those with bilateral OME (P=0.000). Furthermore, children with unilateral AOM were cured more rapidly than were those with bilateral AOM (17). Other reports have shown that the risk of a prolonged course is twice as high in children <2 yr of age with bilateral AOM than in children Ōēź2 yr of age with unilateral AOM (18). However, some reports have claimed that laterality has no effect on the prognosis of OME (19, 20). In the acute stage, unilateral otitis media resolves more quickly than does bilateral otitis media, but the resolution rate of OME decreases abruptly as time passes, regardless of laterality (19, 20). However, in the present study, unilateral OME had a higher resolution rate than did bilateral OME, even though the OME was long-term in nature.
Referred children with OME may require prompt VT insertion. However, we could not pinpoint any factors suggesting prompt or delayed VT insertion, except for laterality. It is highly likely that the duration of OME and medical therapy are important in determining surgical options. Further studies are needed.
CONCLUSIONS
In the tertiary hospital setting, the resolution rate of OME in children was not as high as anticipated, and antibiotics and anti-allergic medications proved to be no more effective than controls for the management of OME. This low resolution rate might be attributable to the pre-treatment characteristics of studied children, such as extended history of OME. Further guidelines, such as those for early VT insertion, may be required in the management of referred children with OME in the tertiary hospital setting. In addition, the importance of meticulous documentation should be emphasized to primary physicians referring pediatric patients with OME.