INTRODUCTION
Diuretics have widely been used in M├®ni├©re's disease to reduce endolymphatic hydrops. There is histological, biochemical, and electrophysiological evidence of the efficacy of osmotic diuretics for this condition [1,2,3,4,5]. Glycerol, an osmotic diuretic, induces transient hearing improvement, and is commonly used for the detection of endolymphatic hydrops [3,6]. However, due to side effects and a rebound increase in the endolymphatic compartment, it is not commonly used therapeutically [7,8].
In contrast, isosorbide, another osmotic diuretic, reduces cochlear endolymph volume without prominent rebound phenomenon. In the guinea pig model with surgically induced endolymphatic hydrops, peak endolymph reduction was observed 6 hours after oral intake [5]. The authors of that study suggested that isosorbide does not enter the endolymph, resulting in the absence of the rebound phenomenon. Clinically, isosorbide has been reported to be effective for relief from symptoms of dizziness, headache, and tinnitus [1]. Further, some cases indicate that hearing improvement is expected after oral isosorbide administration [3,9]. For these reasons, isosorbide has been widely used as a therapeutic agent for M├®ni├©re's disease in Japan [1,10,11].
However, oral administration (PO) of isosorbide has several systemic adverse effects [11,12]. Furthermore, since the intracochlear concentration after administration has not been confirmed, it is difficult to predict therapeutic or adverse effects, which complicates selection of an appropriate dose of isosorbide and results in variable efficacies in different cases.
Therefore, local application of isosorbide, which can deliver higher dose without systemic adverse effects, is considered to be promising for the treatment of M├®ni├©re's disease. However, drug administration through the round window membrane (RWM) into perilymph has not previously been studied. Since isosorbide acts in perilymph by causing a shift of water from the hypo-osmotic endolymph to the hyperosmotic perilymph [5], round window perfusion (RWP) could offer some advantages by delivering the drug directly to the scala tympani (ST) perilymph.
In this study, for the first time, we investigated whether isosorbide can pass into perilymph through RWM, and compared intracochlear isosorbide concentration in the perilymph after PO versus that after RWP.
MATERIALS AND METHODS
Animals and study groups
Sixteen male guinea pigs (32 ears) with a positive Preyer's reflex, weighing 350-400 g, were used. A total of 32 ears were divided into 3 experimental groups: (1) RWP of isosorbide for 15 (n=4), 30 (n=4), and 60 (n=4) minutes; (2) PO of isosorbide and perilymph sampling after 3 hours (n=5) and 6 hours (n=5); and (3) RWP for 30 minutes and perilymph sampling after 3 hours (n=5) and 6 hours (n=5). The study was approved by the Inha University Animal Care and Use Committee (130607-210-1).
RWP and perilymph sampling
The animals were anesthetized with an intraperitoneal injection of ketamine (70 mg/kg) and xylazine (5 mg/kg). Anesthesia was maintained by repeated intramuscular injections of one-fourth to one-third of the initial dose. Using a dorsolateral retro-auricular approach, the bulla was opened using cutting burrs under an operating microscope to expose the round window. Approximately 0.2-0.3 mL of 100% isosorbide solution (KOWA Co., Nagoya, Japan) was administered onto the RWM and the middle ear cavity was filled up. The solution was delivered with a 1-cc syringe fitted with a 30-gauge needle. After the specified perfusion time, residual isosorbide was rinsed out with 10 mL of phosphate buffered saline. After identifying the RWM and the basilar membrane inside of it, ST perilymph was gently harvested in 2-┬ĄL samples through the RWM using an Ultra-fine pipette tip (Multimax 0.1-10 ┬ĄL long-reach tip; Sorenson Bioscience Inc., Salt Lake City, UT, USA) attached to a 10-┬ĄL micropipette (Fig. 1A). To determine the appropriate perfusion time, perilymph was collected at 15, 30, and 60 minutes. In addition, to compare the differences in the isosorbide concentrations between PO and RWP, perilymph was sampled at 3 hours and 6 hours following RWP. The Mann-Whitney U-test was performed to assess statistical differences. A P-value less than 0.05 was considered significant.
PO administration and perilymph sampling
As described previously [5], 4 mL/kg of isosorbide was administered orally by gavage using a feeding tube. Perilymph was collected at 3 hours and 6 hours in the same manner as described above.
Quantitative analysis by high-performance liquid chromatography coupled to refractive index detection (HPLC-RI)
Chromatographic separation and quantification of isosorbide using HPLC analysis was performed. In this step, 2 ┬ĄL of each sample was diluted in 28 ┬ĄL of distilled water and filtered through a 0.2-┬Ąm RC filter (Sartorius Co., Goettingen, Germany). An Acme 9000 HPLC system (YL Instrument Co., Anyang, Korea) equipped with an Aminex HPX-87H Column (Bio-Rad, Hercules, CA, USA) and RI750F RID (YL Instrument Co.) was used for quantification of isosorbide. The mobile phase was 0.005 M sulfuric acid and the flow rate was 0.6 mL/minute. Data acquisition and analysis were performed using the Autochro-3000 chromatography processing program (YL Instrument Co.).
To ensure that perilymph was harvested appropriately without blood contamination, the concentration of glucose in the collected sample was also analyzed (Fig. 1B). With reference to a normal glucose level [13] previously reported in perilymph (average, 3.5 mM; SD, 1.0), samples with a glucose level over 5.5 mM were regarded as contaminated with plasma (average glucose level, 7.3 mM; SD, 0.9) and excluded from the analysis of the difference between PO and RWP.
RESULTS
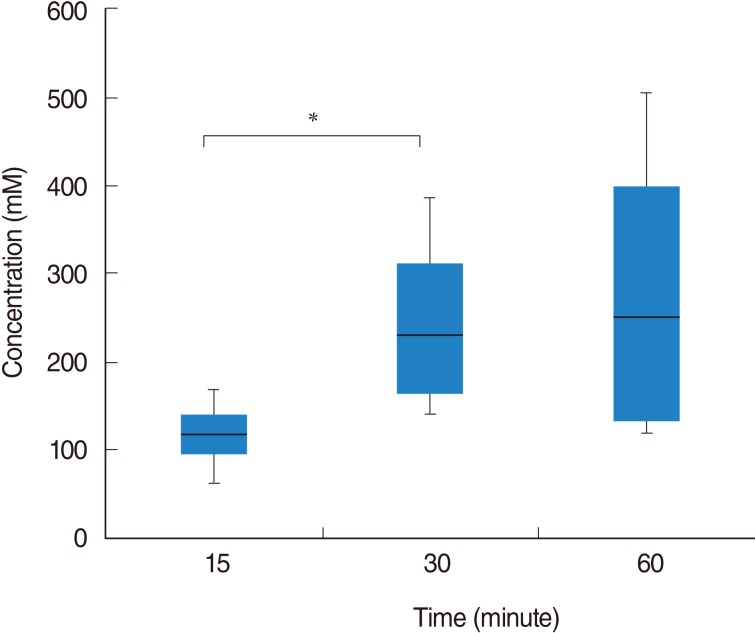
Isosorbide concentration after RWP according to perfusion time
Following RWP for 15 minutes, isosorbide concentration was 116.27┬▒44.65 (SD) mM (range, 62.47 to 168.26 mM). Isosorbide concentrations after RWP for 30 and 60 minutes were 245.48┬▒112.84 mM (range, 140.04 to 385.88 mM) and 279.78┬▒186.32 mM (range, 116.66 to 504.76 mM), respectively. The isosorbide concentration after RWP for 30 minutes was significantly higher than that after RWP for 15 minutes (P=0.043). However, there was no significant difference between the concentrations recorded after RWP for 30 and 60 minutes (P=0.773) (Fig. 2).
Isosorbide concentrations 3 and 6 hours following RWP versus PO
In all 20 samples, isosorbide was detected by HPLC. However, five samples were excluded because of high glucose levels, which were suspicious of blood contamination. Of the PO samples, 1 sample harvested at 3 hours showed a glucose level of 7.2 mM. Similarly, among the RWP samples, 2 samples at 3 hours and 6 hours each showed high glucose levels (range, 6.02 to 7.16 mM). Thus, a total of 15 samples were analyzed, with a mean glucose level of 4.26 mM (median, 4.15; range, 3.29 to 5.49 mM).
At 3 and 6 hours after PO, isosorbide concentrations were 28.88┬▒4.69 mM (range, 25.19 to 34.74 mM) and 12.67┬▒2.28 mM (range, 10.41 to 15.14 mM), respectively. In contrast, isosorbide concentrations were 117.91┬▒17.70 mM (range, 104.65 to 138.01 mM) and 75.03┬▒14.82 mM (range, 63.73 to 91.80 mM) at 3 and 6 hours after RWP for 30 minutes, respectively. Isosorbide concentrations in ST perilymph following RWP were significantly higher than those following PO at both 3 hours and 6 hours (P=0.025, P=0.034, respectively) (Fig. 3). While the concentration was 245.48┬▒112.84 mM immediately after RWP for 30 minutes, it decreased by 52% in 3 hours (117.91┬▒17.70 mM) and by 70% in 6 hours (75.03┬▒14.82 mM) (Figs. 2, 3).
DISCUSSION
There is experimental [1,2,3,4,5] and clinical [10,11] evidences that isosorbide is one of effective drugs for M├®ni├©re's disease. Its dehydrating effect was microscopically confirmed in the guinea pig model with surgically induced hydrops. Specifically, reduction of endolymph was shown histologically in the hydrops model at 3 hours and 6 hours after PO administration [5]. In addition, isosorbide has been reported to cause no rebound phenomenon, in contrast to glycerol [7]. For these reasons, oral isosorbide has been used for the treatment of M├®ni├©re's disease in Japan and Korea. However, since the intracochlear concentration after oral intake of isosorbide has not been studied, the appropriate therapeutic dose is not clear yet.
In our study, we measured isosorbide concentration in perilymph after PO of isosorbide for the first time. In a previous study [5], a peak reduction of cochlear endolymph volume occurred 6 hours after oral intake in the animal model. In this study, however, intracochlear concentration was higher at 3 hours than at 6 hours. This suggests that a time delay exists between the peak concentration of isosorbide and the peak reduction of endolymph resulting from intracochlear fluid shift.
Oral isosorbide treatment, though, has several problems. First, the effective intracochlear concentration has not been studied, and may vary across patients after oral intake. Second, PO isosorbide has systemic adverse effects. Some patients have complained of nausea, vomiting, or headache [11]. Furthermore, isosorbide may increase antidiuretic hormone levels and osmolality in plasma, which may worsen endolymphatic hydrops over the long term [12]. As a result, it is difficult to recommend a high oral dose of isosorbide.
Intratympanic drug injection has been widely used for the treatment of M├®ni├©re's disease, including dexamethasone and gentamicin therapy [14]. Since local application decreases systemic adverse effects and allows the use of a higher dose of medication, RWP procedures such as intratympanic injection could be another option for isosorbide treatment. However, isosorbide permeability through RWM and its concentration in perilymph has not been previously investigated. In this study, we found that isosorbide can pass across the RWM, and we also measured intracochlear concentration after RWP of isosorbide.
The RWM acts like a semipermeable membrane, of which permeability depends on size, configuration, concentration, liposolubility, and electrical charge of substances [15]. Compared to gentamicin and dexamethasone that can diffuse through RWM, isosorbide has smaller size (0.76 nm in major diameter) and low molecular weight (146.14 g/mol) [5], which are advantageous for diffusion. However, other factors of isosorbide affecting permeability through RWM need to be studied.
Intracochlear isosorbide concentration following RWP was much higher than that following PO. At 3 hours after administration, RWP resulted in a concentration over 4 times greater than that resulting from oral intake. Similarly, at 6 hours after administration, RWP resulted in a concentration over 7 times higher than that resulting from oral intake. Thus, RWP can deliver a higher dose of isosorbide than PO, without the concerns of systemic adverse effects.
In our study, RWP also resulted in a rapider increase in intracochlear concentration of isosorbide, when compared to the increase noted after PO. Even after RWP for a short time of 15 minutes, mean intracochlear concentration was 116.27 mM, which was over 4 times higher than the concentration at 3 hours after PO. This rapid action suggests RWP could be appropriate for the treatment of an acute attack of M├®ni├©re's disease.
Additionally, we investigated the appropriate perfusion time of RWP. RWP for 30 minutes resulted in a significantly higher concentration than that obtained with RWP for 15 minutes. Interestingly, RWP for 30 minutes showed lower standard deviation than that for 60 minutes. We assume that leak through the Eustachian tube might cause the inconsistency in the group of RWP for 60 minutes. In addition, perfusion for 30 minutes is clinically applicable and acceptable for intratympanic drug injection. Thus, we suggest that RWP for 30 minutes is considered to be appropriate for future studies in guinea pigs.
Even though we found that RWP of isosorbide could be feasible for treatment of M├®ni├©re's disease, further study is necessary on several aspects. First, we sampled the perilymph through the RWM from the basal cochlear turn in order to confirm the permeability of isosorbide through the RWM into the ST. This procedure can exclude the drug delivery through the oval window and enable sampling perilymph immediately after the RWP.
However, this can result in the contamination of cerebrospinal fluid through cochlear aqueduct [16]. In addition, drug concentrations after RWP could be higher in the basal turn of the cochlea than in the apical turn at the early stage, as reported for intratympanic injection of dexamethasone [17] and gadolinium [18]. Thus, in order to analyze base-to-apex concentration gradients through whole perilymph and to investigate the time course and duration of isosorbide action, it might be preferable to sample perilymph serially from the apex of the cochlea with minimal damage as described by Hahn et al. [17]. Second, this study was performed in normal guinea pigs. In order to ensure the efficacy of RWP, further studies are necessary, not only of isosorbide concentration in perilymph, but also of endolymph reduction in vivo in animal models with hydrops. Third, the safety of RWP for middle ear mucosa or inner ear structure should be assessed through functional and histologic studies before clinical use. Moreover, the further investigation of serum isosorbide concentration might be necessary with regards to the systemic adverse effects after RWP, though the drug leakage through Eustachian tube could be negligible because the drug dose of RWP is very lower (0.2 mL) than that of PO.
In conclusion, isosorbide can pass through the RWM into the perilymph after RWP, and RWP of isosorbide showed rapider action and higher intracochlear concentration than PO.