|
 |
- Search
AbstractObjectivesTo compare tinnitus patients who have normal hearing between 250 Hz and 8 kHz with normal controls with regard to the ability of each group to hear extended high-frequency pure tone thresholds.
MethodsWe enrolled 18 tinnitus patients, each of whom had a threshold of HL <25 dB and threshold differences of <10 dB between ears at frequencies of 250 and 500 Hz and 1, 2, 4, and 8 kHz. We also enrolled age- and gender-matched normal volunteers (10 ears), for each patient. Extended high frequency pure tone audiometry was performed, and the mean hearing thresholds at 10, 12, 14, and 16 kHz of each tinnitus ear were compared with those of the 10 age- and sex-matched normal ears.
ResultsOf the 18 patients with tinnitus, 12 had significantly increased hearing thresholds at more than one of the four high frequencies, compared with the normal group. When we assessed results according to frequency, we found that 8 patients had decreased hearing ability at 10 kHz, 10 at 12 kHz, 8 at 14 kHz, and 4 at 16 kHz.
In most cases, tinnitus is accompanied by decreased hearing ability, suggesting that the peripheral acoustic organs are a site of occurrence of tinnitus (1, 2). Severity of tinnitus is often found to increase with the degree of hearing loss and the pitch of tinnitus often coincides with the frequency of the lesion or is just below the precipitous edge of the lesion. These findings demonstrate that damage to cochlear hair cells plays a crucial role in the pathogenesis of tinnitus (3-7). However, tinnitus can also appear in deaf patients or those in which the auditory nerve has been surgically sectioned. Studies measuring changes in positron emission computed tomography (CT) associated with tinnitus have supported the hypothesis that tinnitus originates in the central nervous system. Thus, tinnitus does not develop from damaged hair cells, but is a phantom perception caused by plastic readjustments of the auditory center due to decreased afferent signals (8-10). In other words, in patients with hair cell destruction within a specific frequency range, there is a decrease in signal transduction of the corresponding frequency from the acoustic nerve. This reduced nerve input, which has a characteristic frequency in the hearing loss range, may result in a reduction of lateral inhibition at the dorsal cochlear nucleus or inferior colliculus. The reduced nerve input may also be due to the development of tinnitus caused by the hypersensitivity and hyperactivity in auditory neurons at the edge frequency (10, 11). In addition, tinnitus may be caused or changed by somatosensory or somatic motor nerves other than the acoustic nerve, suggesting that tinnitus may occur in the dorsal cochlear nucleus (11, 12). Tinnitus can be modulated by various somatosensory manipulations, and cross-modality interactions between auditory and somatosensory information can take place in the dorsal cochlear nucleus.
The occurrence of tinnitus, together with normal hearing ability, suggests that the former may develop purely in the central nervous system, with no relation to cochlear damage. The proportion of patients with tinnitus and normal hearing has been reported to range from 7.4% to 20% (13-15). These studies, however, are based on classical pure tone audiometry, with frequencies that do not exceed 8 kHz. Because audible frequencies range between 20 Hz and 20 kHz in humans (16), we hypothesized that the proportion of tinnitus patients with completely intact cochlea is between 7.4% and 20%. We therefore measured hearing thresholds at extended high frequencies in patients with tinnitus who had normal hearing on classical pure tone audiometry within the range 250 Hz to 8 kHz. If there are tinnitus patients with complete normal hearing at the highest end of the audible hearing frequency, tinnitus may originate in the central nervous system. The purpose of this study was to evaluate how many patients who have conventionally normal hearing reveal abnormal hearing thresholds at extended high-frequencies.
Of 510 patients who visited our clinic between January 2006 and May 2008 with a chief complaint of subjective tinnitus, 49 had a pure tone threshold <25 dB HL and threshold differences <10 dB between ears at frequencies of 250 and 500 Hz and 1, 2, 4, and 8 kHz. Patients with objective tinnitus, abnormal otologic findings, or a history of neurological illness or endocrine diseases such as hypothyroidism or diabetes, were excluded. Of these patients, however, 31 were ineligible for this study because their symptoms disappeared within 3 months, they refused to participate, or they could not be followed-up. Thus, 18 patients (18 ears), each of whom had persistent tinnitus for a minimum of 3 months, were enrolled in this study. This group consisted of 3 men and 15 women with a mean age of 41.2 yr (range, 31 to 54 yr). All 18 had normal waves and latencies in 30 dB nHL click stimuli of auditory brainstem response. Tinnitus was present in 7 left ears and 11 right ears; none had bilateral tinnitus. For each patient, we enrolled 5 age- and sex-matched normal volunteers (10 ears), each of whom had a threshold <25 dB HL and threshold differences <10 dB between ears at frequencies below 8 kHz. Mean hearing threshold was measured over an extended high frequency range, including frequencies of 10, 12, 14, and 16 kHz. This was done for each of the 18 patients with tinnitus and for 5 volunteers (10 ears) matched in age and sex to each of the 18 patients. Because there were 2 pairs of patients of the same age and gender (patients 5 & 6, 15 & 16) (Table 1), a total of 160 control ears were enrolled. Normal individuals were excluded if there were complaints of subjective tinnitus, a family history of hearing difficulty, a previous history of noise exposure or otic diseases, or complaints of a subjective decrease in hearing ability. We used a pure tone audiometer (Orbiter 922; GN Otometrics, Copenhagen, Denmark) and a specialized headphone for the ultra-high frequency range (HAD-200; Sennheiser, Wedemark, Germany) to obtain hearing thresholds for frequencies of 10, 12, 14, 16, 18, and 20 kHz at a dB sound pressure level. Measurements were done using an ascending-descending technique, in 5 dB steps at all frequencies. If a patient made two or more responses to a set of 3 stimuli, she/he was deemed to have heard the sound. At frequencies >18 kHz, most patients did not respond to the maximum stimulatory sound, even among volunteers without tinnitus. We were not provided with any calibration form from the manufacturing company. Thus, we compared the mean hearing thresholds of each tinnitus ear and 10 normal ears at 10, 12, 14, and 16 kHz using a Mann-Whitney test. The Mann-Whitney test and Fisher's exact test were used for comparisons at each frequency between the tinnitus group and the control group (stratified by age-decade, and the characteristics of tinnitus).
Of the 18 patients with tinnitus, 12 (patients 1, 2, 3, 4, 6, 8, 10, 11, 12, 13, 14, and 17) had significantly increased hearing thresholds at more than one of the three test frequencies in comparison with the age- and sex-matched normal group. In the other 6 patients (patients 5, 7, 9, 15, 16, and 18), the hearing threshold did not differ or was lower than that of the normal group. Of the 12 patients with increased hearing threshold at extended high frequencies, three (patients 6, 13, and 14) had decreased hearing at 10 and 12 kHz, five (patients 2, 8, 10, 11, and 12) had decreased hearing at 10, 12, and 14 kHz, two (patient 1 and 4) had decreased hearing at 12, 14, and 16 kHz, one (patient 17) had decreased hearing at 14 and 16 kHz, and one (patient 3) had decreased hearing at 16 kHz. When we assessed results according to frequency, we found that 8 patients had decreased hearing ability at 10 kHz, 10 had decreased hearing at 12 kHz, 8 had decreased hearing at 14 kHz, and 4 had decreased hearing at 16 kHz (Figs. 1-4).
In the comparison between the mean of hearing thresholds of tinnitus subjects (n=18) with <25 dB HL at the conventional frequencies, and that of their counterparts (subjects without tinnitus, n=160) in dB sound press level (SPL) by age-decade, tinnitus subjects had a significantly higher threshold at 10 kHz only. The mean hearing thresholds in the tinnitus patients group aged <35 yr, and in the group aged 35-44 yr were significantly higher than those of normal subjects at 14 kHz and 10 kHz, respectively. However, there was no significant difference between the two groups at all frequencies for those aged >45 yr (Table 2).
In the comparison of characteristics between tinnitus patients within the normal hearing threshold up to extended high frequencies and tinnitus patients with increased hearing thresholds at extended high frequencies, there was no significant difference (Table 3).
Of the 510 tinnitus patients who visited our clinic, 49 (9.6%) had normal hearing on conventional audiometry <8 kHz, which is within the range (7.4 to 20%) previously reported (13-15). Of the 18 enrolled patients with tinnitus, all of whom had normal hearing at frequencies below 8 kHz, 12 (66.7%) had decreased hearing ability at more than one of the four extended high frequencies, 10, 12, 14, and 16 kHz. These results indicate that there are patients with tinnitus who are found to be normal on conventional audiometry, with a threshold below 8 kHz, who can be excluded from the current analysis if extended high frequency stimuli are included. In contrast to our findings, an analysis of extended high frequency thresholds in 17 tinnitus patients aged 17 to 45 yr and in 17 age- and sex-matched controls found no significant between group differences (14). The earlier study, however, compared patients and controls at a ratio of 1:1. Because there are great discrepancies in extended high frequency thresholds among individuals, the hearing threshold of each control likely does not represent the corresponding age group (17). Moreover, it would be rather inaccurate to make comparisons based on mean threshold values of whole patients and controls, and not make comparisons between hearing thresholds of individual patient and matched controls, which would show great age- and sex-related discrepancies. As age increases, such factors as noise exposure, accumulation of ototoxic materials and aging itself will lower extended high frequency hearing ability, making it very difficult to establish a certain level of hearing threshold as the normal value. Thus, to reduce inter-individual differences in extended high frequency hearing ability, we matched 5 volunteers (10 ears) by age and sex to each of our 18 patients. Standard deviations of hearing threshold at 10, 12, and 14 kHz in the 160 normal ears were 12.6, 20.6, and 22.2, respectively. These results did not differ greatly from earlier studies, which showed that the standard deviations at these three extended high frequencies were 11, 15.6, and 20.2 dB respectively, in 104 ears of patients aged 18 to 49 yr (17). We found that the standard deviation was greater at higher frequencies, which may be due to the greater effect on the anatomical structure of the external acoustic meatus (18). In addition, because the mean age of normal volunteers was relatively high (41.2 yr [range, 31 to 54 yr]) and age-induced hearing impairments may have already occurred, the threshold of normal individuals had greater standard deviations at higher frequencies. However, in the case of 16 kHz, too many cases of "106 dB SPL or more" (cases of no responses to the maximal stimulatory sound) induced such a small standard deviation (12.0).
Of the 18 patients with tinnitus, 6 did not show a decrease in hearing ability at extended high frequencies, suggesting that they could have normal hearing at all audible frequencies. The pathogenesis of tinnitus where there is no decrease in hearing ability may have various causes. Tinnitus may be induced purely in the central nervous system without damage to peripheral sensory organs. In most patients with tinnitus, the afferent signals are decreased by damage to peripheral sensory organs, and plastic changes may follow in a specific area of the acoustic center, which may secondarily induce spontaneous activity. However, a decrease in afferent acoustic signals is not essential (11, 19). For example, in individuals with somatic tinnitus syndrome, the somatic manipulations may stimulate a specific area of the acoustic center. This may lead to tinnitus, which occurs regardless of hearing ability (12). In patients without a concurrent decrease in hearing ability, damage to the hair cells may be mild or at a very early stage, although it may be present in peripheral sensory organs. Biochemical changes preceding structural damage to the hair cells may induce tinnitus (20). Noise exposure and specific pharmacologic agents can elevate intracellular Ca2+ concentration, resulting in elongation of the outer hair cells and decreases in their stiffness. This may distort the relationship between the inner hair cells and the tectorial membrane and may result in easy deflection of the stereocilia, causing increased activity in the afferent cochlear nerve and resulting in tinnitus (21). In addition, the excessive release of glutamate from the terminus of the internal hair cells or hyperactivity of N-methyl-D-aspartate receptors in the spiral ganglion can induce excessive activation of the auditory nerve. Following recovery of hair cells from biochemical injury, tinnitus may persist as a phantom phenomenon in the acoustic center (22). For example, tinnitus may also persist following the recovery of hearing ability from the transient threshold shift due to acoustic trauma.
In the comparison between the mean of hearing thresholds of the tinnitus groups and the control groups at each frequency by age-decade, we found a difference at each frequency, excluding subjects aged >45 yr. Though there was a difference in one frequency only, some tinnitus patients could have damage in any small portion of cochlea. For those aged >45 yr, no significant difference between the tinnitus and non-tinnitus groups at all frequencies was found to have been caused by progressive age-induced hearing impairments.
We also tried to compare the characteristics of tinnitus between tinnitus patients within the normal hearing thresholds up to extended high frequencies and patients with increased hearing thresholds at extended high frequencies. However the numbers in the samples were too small to make any meaningful conclusion.
We also attempted to measure hearing thresholds at 18 and 20 kHz. However, we noted that no reactions occurred at frequencies of 18 kHz and above in normal volunteers without tinnitus. We could not, therefore, measure differences in individuals with and without tinnitus at hearing thresholds >16 kHz. Thus, it may be difficult to determine whether tinnitus combined with completely intact cochlea or minimal damage without a concurrent decrease in hearing ability manifests or not. Even patients with tinnitus who showed normal hearing at frequencies up to 16 kHz could not be determined to be normal at all audible frequencies. We also do not know about extended low frequencies (<250 Hz), because the tests were not conducted. This can be considered a limitation caused by testing equipment.
In this study, decreased hearing ability was seen at ultra highfrequencies in some patients with tinnitus who had normal hearing below 8 kHz. Thus, the proportion of patients with tinnitus who have normal hearing within the entire audible range is smaller than in previous reports.
References1. Kiang NY, Moxon EC, Levine RA. Auditory-nerve activity in cats with normal and abnormal cochleas. In: Sensorineural hearing loss. Ciba Found Symp. 1970. p. 241-273.
2. Hazell JW, Jastreboff PJ. Tinnitus. I: Auditory mechanisms: a model for tinnitus and hearing impairment. J Otolaryngol. 1990 2;19(1):1-5. PMID: 2179573.
3. Alberti PW. Tinnitus in occupational hearing loss: nosological aspects. J Otolaryngol. 1987 2;16(1):34-35. PMID: 2951528.
4. Coles A. In: Vernon JA, Moller AR, editors. Classification of causes, mechanisms of patient disturbance, and associated counseling. Mechanisms of tinnitus. 1995. Needhan Heights (MA): Allyn & Bacon; p. 11-19.
5. Eggermont JJ, Sininger Y. In: Vernon JA, Moller AR, editors. Correlated neural activity and tinnitus. Mechanisms of tinnitus. 1995. Needham Heights (MA): Allyn & Bacon; p. 21-34.
6. Ochi K, Ohashi T, Kenmochi M. Hearing impairment and tinnitus pitch in patients with unilateral tinnitus: comparison of sudden hearing loss and chronic tinnitus. Laryngoscope. 2003 3;113(3):427-431. PMID: 12616191.
7. Weiss AD, Weiss ER. Acoustic trauma: tinnitus and vertigo. J Laryngol Otol. 1984;(Suppl 9):82-83.
9. Lockwood AH, Salvi RJ, Coad ML, Towsley ML, Wack DS, Murphy BW. The functional neuroanatomy of tinnitus: evidence for limbic system links and neural plasticity. Neurology. 1998 1;50(1):114-120. PMID: 9443467.
11. Kaltenbach JA, Zhang J, Finlayson P. Tinnitus as a plastic phenomenon and its possible neural underpinnings in the dorsal cochlear nucleus. Hear Res. 2005 8;206(1-2):200-226. PMID: 16081009.
12. Levine RA. Somatic (craniocervical) tinnitus and the dorsal cochlear nucleus hypothesis. Am J Otolaryngol. 1999;Nov–Dec;20(6):351-362. PMID: 10609479.
13. Sanchez TG, Medeiros IR, Levy CP, Ramalho Jda R, Bento RF. Tinnitus in normally hearing patients: clinical aspects and repercussions. Braz J Otorhinolaryngol. 2005;Jul–Aug;71(4):427-431. PMID: 16446955.
14. Barnea G, Attias J, Gold S, Shahar A. Tinnitus with normal hearing sensitivity: extended high-frequency audiometry and auditory-nerve brain-stem-evoked responses. Audiology. 1990;29(1):36-45. PMID: 2310352.
15. Jastreboff PJ, Jastreboff MM. Tinnitus retraining therapy for patients with tinnitus and decreased sound tolerance. Otolaryngol Clin North Am. 2003 4;36(2):321-336. PMID: 12856300.
16. Cutnell JD, Johnson KW. Physics. 1998. 4th ed. NewYork: Wiley.
17. Ahmed HO, Dennis JH, Badran O, Ismail M, Ballal SG, Ashoor A, et al. High-frequency (10-18 kHz) hearing thresholds: reliability, and effects of age and occupational noise exposure. Occup Med (Lond). 2001 6;51(4):245-258. PMID: 11463869.
18. Ravicz ME, Olson ES, Rosowski JJ. Sound pressure distribution and power flow within the gerbil ear canal from 100 Hz to 80 kHz. J Acoust Soc Am. 2007 10;122(4):2154-2173. PMID: 17902852.
19. Saunders JC. The role of central nervous system plasticity in tinnitus. J Commun Disord. 2007;Jul–Aug;40(4):313-334. PMID: 17418230.
20. Henry JA, Dennis KC, Schechter MA. General review of tinnitus: prevalence, mechanisms, effects, and management. J Speech Lang Hear Res. 2005 10;48(5):1204-1235. PMID: 16411806.
21. Sziklai I. The significance of the calcium signal in the outer hair cells and its possible role in tinnitus of cochlear origin. Eur Arch Otorhinolaryngol. 2004 11;261(10):517-525. PMID: 15609110.
22. Sahley TL, Nodar RH. A biochemical model of peripheral tinnitus. Hear Res. 2001 2;152(1-2):43-54. PMID: 11223280.
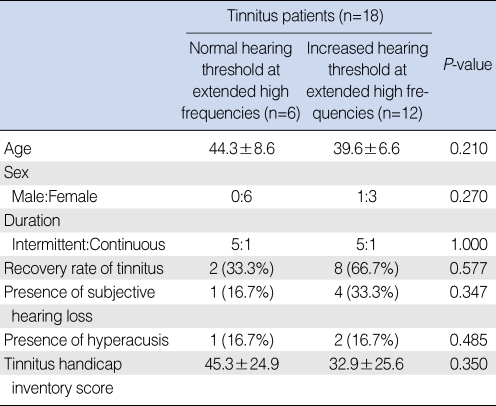
Fig. 1Hearing thresholds of tinnitus patients and controls at 10 kHz. Eight patients (patients 2, 6, 8, 10, 11, 12, 13, and 14) had decreased hearing ability at 10 kHz (*P<0.05) (○: controls, □: patient). 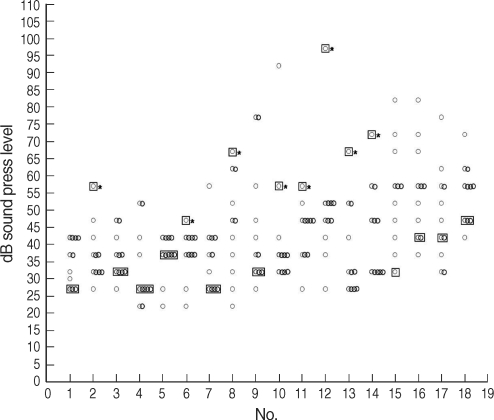
Fig. 2Hearing thresholds of tinnitus patients and controls at 12 kHz. Ten patients (patients 1, 2, 4, 6, 8, 10, 11, 12, 13, and 14) had decreased hearing at 12 kHz (*P<0.05) (○: controls, □: patient). 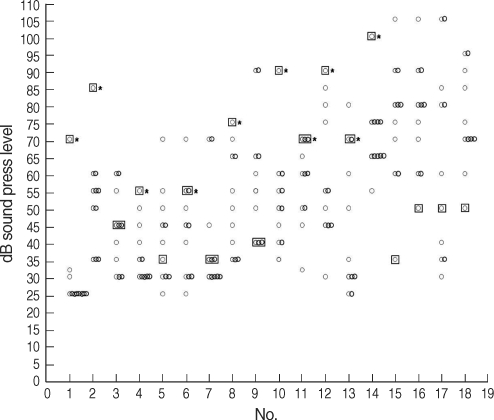
Fig. 3Hearing thresholds of tinnitus patients and controls at 14 kHz. Eight patients (patients 1, 2, 4, 8, 10, 11, 12, and 17) had decreased hearing at 14 kHz (*P<0.05) (○: controls, □: patient). 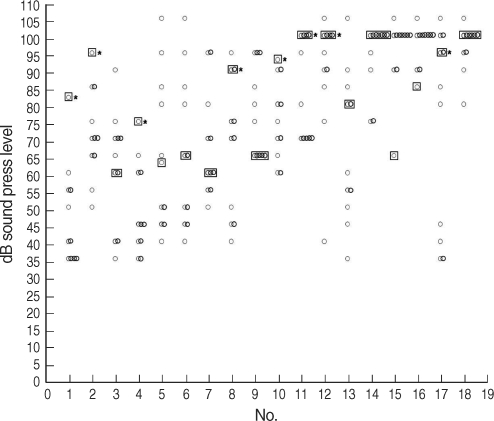
Fig. 4Hearing thresholds of tinnitus patients and controls at 16 kHz. Four patients (patients 1, 3, 4, and 12) had decreased hearing at 16 kHz (*P<0.05) (○: controls, □: patient). 
Table 1Extended high-frequency threshold in tinnitus patients and corresponding controls 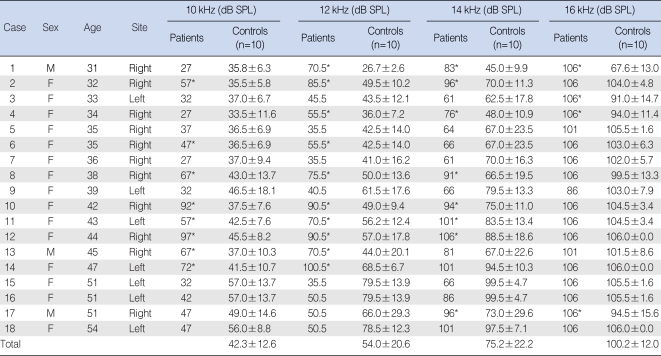 Of the 18 patients with tinnitus, 12 (patients 1, 2, 3, 4, 6, 8, 10, 11, 12, 13, 14, and 17) had significantly increased hearing thresholds at more than one of the three tested frequencies in comparison with the age- and gender-matched normal group. In the other six patients (patients 5, 7, 9, 15, 16, and 18), the hearing threshold did not differ or was lower than that of the normal group. *P<0.05. SPL: sound press level. |
|
|||||||||||||||||||||||||||||||||||||||