CASE REPORT
A 72-year-old woman presented with a three-month history of rapid-growing mass in the lower anterior neck. She complained of hoarseness and dysphagia, but was not dyspneic. She had been diagnosed with a benign cystic thyroid nodule 7 years previously, for which she underwent percutaneous alcohol injection a year later. She has been taking synthetic thyroid hormone replacement for 3 years. Her past medical history included diabetes mellitus for fifteen years, managed by oral hypoglycemic agents.
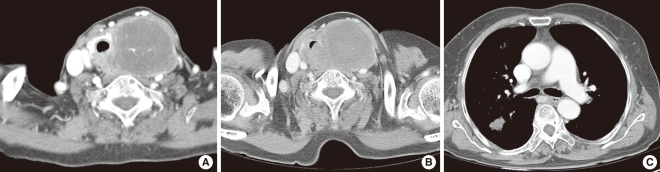
Physical examination revealed a 6 cm, firm, fixed, and non-tender mass in the lower anterior neck, with no mass was palpable in either lateral neck region. Flexible endoscopic laryngeal examination demonstrated left vocal cord palsy. Fine needle aspiration cytology of the mass demonstrated cystic change. Ultrasound scan showed a 6├Ś5 cm-sized, heterogeneously hypoechoic nodule with internal calcification in the left lobe of the thyroid gland. Computed tomography (CT) scan revealed a large, low-attenuating mass replacing the left thyroid gland extending to the level of the hyoid bone superiorly and superior mediastinum inferiorly with possible invasion to the trachea. The mass displaced the trachea to the right and anteriorly, and the internal jugular vein laterally, while compressing the lumen (Fig. 1A). No metastatic lesions or other primary foci were detected. Esophagogram showed extrinsic compression but no direct invasion to the esophagus.
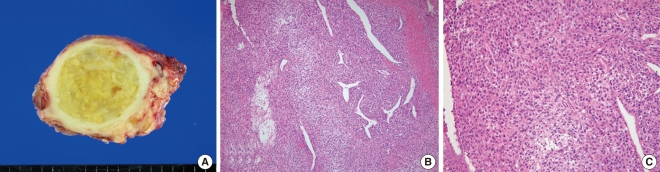
Under the impression of thyroid carcinoma, we planned to perform total thyroidectomy with tracheal resection and end-to-end anastomosis, which would be converted to an incisional biopsy and tracheotomy if the frozen biopsy showed anaplastic carcinoma. Two weeks after the initial visit, and two days before the planned operation, the patient presented to an emergency room due to dyspnea. Neck CT scan showed tumor tracheal invasion and airway narrowing (Fig. 1B), while chest CT scan revealed multiple variable sized nodular lesions in both lung fields, suggestive of lung metastasis (Fig. 1C). In view of the findings, total thyroidectomy with tracheal fenestration was performed for the purpose of biopsy and palliation. Intraoperatively, the main foci of the tumor within the thyroid gland was found to be infiltrating to the surrounding thyroid tissue and was firmly attached to the internal jugular vein, esophagus and trachea, as well as exerted intraluminal extension to the trachea. Frozen biopsy demonstrated unspecific sarcoma. The resected mass measured about 6├Ś5├Ś4.5 cm. The cut surface was yellowish white, heterogeneous and focally myxoid (Fig. 2A). Histologically, the tumor was consisted of fascicles and sheets of dense, uniform, relatively small ovoid neoplastic cells. The tumor displayed a hemangiopericytic vascular pattern. However, epithelial component was not identified (Fig. 2B and C). Immunohistochemical staining for CD 99 was strongly positive while negative for cytokeratin, desmin, S-100, CD 31, CD 34, and epithelial membrane antigene (EMA). Molecular genetic analysis of the SYT/SSX fusion gene transcript was positive, confirming the diagnosis of SS. The postoperative course was uneventful. Concurrent chemoradiation had been planned. However, she failed to return for follow-up following discharged, and died 2 months after the operation due to unknown causes.
DISCUSSION
SS is a pleuripotential mesenchymal malignant tumor that comprises of 10% of all soft tissue sarcoma. Most SS arise in the extremities, near the large joints, but do not originate from synovial tissues. Therefore, they can also occur in any other anatomical location, including the head and neck, abdominal wall, and the thoracic cavity (4). SS arising in the head and neck account for 10% of all SS, and mostly occurs in the hypopharynx and retropharynx (1). Only 2 cases of SS originating from the thyroid gland have been reported in the English literature (2, 3). Clinical signs and symptoms vary with tumor location. For example, a growing neck mass is often associated with signs of compression, dysphagia, and hoarseness (5). Our patient experienced a rapidly growing anterior neck mass with signs of airway compression and left vocal cord paralysis.
With regard to the origin of SS in our case, after reviewing the clinical and pathologic findings of the patient's tumor, we concluded that the tumor originated from the thyroid gland, rather than from the adjacent or distant organs.
In cases of SS originating from other neck regions, the main tumor foci are usually situated around their local regions, distinct from the thyroid gland (6). In our case, however, imaging studies and intraoperative findings showed that the tumor had replaced the surrounding thyroid tissue and extended to the adjacent structures, including the internal jugular vein, the esophagus, and trachea. Thorough review of the pathologic studies were consistent with our clinical findings. It is impossible to define the tissue of origin of this tumor more precisely, due to near complete involvement of the thyroid gland and prior injection of alcohol to the thyroid nodule had destroyed the thyroid parenchyma.
SS typically affects adolescents and young adults. Less than 10% of SS patients is over 60 years old (4). The age of the two previously reported primary thyroid SS patients was 60, suggestive that SS of the thyroid gland tends to develop at an older age, in contrast to SS that originate from other locations (2).
According to histologic findings, SS can be classified as biphasic or monophasic. Biphasic SS is consisted of spindle and epitheloid cells which rarely causes diagnostic difficulties. On the other hand, monophasic SS can exhibit variable patterns and present as diagnostic challenge. Monophasic SS should be distinguished from other spindle cell sarcomas, such as leimyosarcoma, rhabdomyosarcoma, fibrosarcoma, and malignant peripheral nerve sheath tumor (MPNST) (7, 8). Differential diagnoses should also include carcinosarcoma or spindle epithelial tumor with thymus-like differentiation (SETTLE) (1). Our case showed an atypical histologic pattern comprised of short spindle cells surrounded by collagenous matrix, which rendered it difficult to distinguish from other spindle cell malignancies.
Immunohistochemistry often yields conflicting results when the histopathologic pattens of SS are atypical. Typically, SS reacts positively to epithelial markers (EMA, cytokeratin), and negatively to non-epithelial markers such as CD 34. The expression of epithelial markers is observed in some cases of MPNST, anaplastic carcinoma and carcinosarcoma (1, 8). The expression of S-100 protein and CD 99 is observed in some cases of SS. Bcl-2 is widely expressed in the spindle cells, which helps differentiation from other spindle cell tumors (5). In our case, the tumor showed more conflicting results, reacting positively only to CD 99 and negatively to EMA, cytokeratin, desmin, and CD34. We did not perform Bcl-2 immunostaining. However, molecular study for SYT/SSX fusion transcript - the most accurate diagnostic method - confirmed SS. Both classical and molecular analyses therefore represent particularly useful diagnostic techniques that help avoid diagnostic confusion.
The primary treatment of SS is wide surgical excision to obtain tumor-free margins, because most soft tissue malignancies tend to extend along the fascia. However, this cannot always be accomplished in the head and neck region because of the tumor's proximity to vital structures. Therefore, a combination of surgery and postoperative radiotherapy is generally recommended. Chemotherapy based on ifosfamide may also be considered as adjuvant treatment or for the treatment of recurrent tumor (1). As described in previous and the current reports, SS arising from the thyroid gland tends to be infiltrative and aggressive, necessitating early and comprehensive treatment (2).
In summary, primary SS arising from the thyroid gland is extremely rare, and thus, the clinical course remains unclear. In our case, histologic findings were not typical of SS and the detection of SYT-SSX fusion gene transcript was very helpful for diagnosis. Our case resembled anaplastic thyroid carcinoma with extensive local invasion, rapid progression, and early distant metastasis. Because of the aggressive nature of this tumor, early diagnosis and comprehensive treatment including wide resection and adjuvant chemoradiation is required.