Wang: Significance of Susceptible Gene Expression Profiles in Nasal Polyposis
Abstract
Nasal polyposis (NP) is a common chronic inflammatory disease of the rhinosinus mucosa and a complex disease with strong genetic and environmental components. During the past 10 to 20 yr, many studies have been performed to determine differential gene expression profiles between NP and normal nasal tissues, in order to identify susceptible genes that are associated with NP-related traits. Despite achievement in the identification of candidate genes and their associated pathogenic pathways, the large challenges remain as the genetic and molecular alterations required for its development and progression are still unclear. Therefore, the development of novel, powerful tools for gene discovery, and a closer integration of genetics and medical biology would provide valuable insight into the pathogenesis of NP.
Keywords: Nasal polyposis; Genetics; Gene expression profile; Pathogenesis and pathophysiology; Susceptible genes and pathways
INTRODUCTION
Nasal polyposis (NP) is one of the most common mass lesions of the nose and was first described 4,000 yr ago in ancient Egypt. The prevalence of NP is reported from 0.2% to 4.3% worldwide, with a ratio of 2-3:1 between males and females ( 1). In children, NP is relatively rare and has a close relationship with asthma and cystic fibrosis ( 2). NP is a multifactorial condition which is often associated with many diseases and pathogenic disorders, such as allergy, infection, cystic fibrosis, asthma, and aspirin intolerance ( 1). However, the underlying mechanisms interlinking these pathologic conditions to NP formation remain unclear. NPs are outgrowths of nasal mucosa which are smooth, semitranslucent, gelatinous and pale. Histo-morphological characterization of polyp tissue reveals frequent epithelial damage, a thickened basement membrane, and oedematous to sometimes fibrotic stromal tissue, with a reduced number of vessels and glands, but virtually no neural structure ( 3). Hellquist ( 4) divided NPs into four histological patterns. The most common type was the edematous NP with predominant eosinophilia, which constituted 85-90% of NPs. Other types include fibroinflammatory, hyperplasia of seromucinous glands and atypical stroma. It is not clear whether this classification has an impact on the differentiation of pathogenic mechanisms and clinical management of NPs. Although the mechanisms involved in the pathogenesis of NP remain largely unclear, there are reports suggesting an underlying genetic predisposition. This concept is supported by some clinical data and genetic studies. This paper reviews recent understanding of the pathogenic mechanisms of NPs, which are influenced by a complex immune procedure including interaction of multiple genes. This review paper does not include NP in cystic fibrosis (CF), which is known to be a hereditary disease with multi-systemic involvement with genetic variations, presenting with defect in chloride transport across membranes and dehydrated secretions.
FAMILY AND TWIN STUDIES OF NPS
An interesting observation is that NP is frequently found to run in families, suggesting a hereditary or shared environmental factor. In the study by Rugina et al. ( 5), more than half of 224 NP patients (52%) had a positive family history of NP. The presence of NP was considered when NP had been diagnosed by an ENT practitioner or the patients had undergone sinus surgery for NP. A lower percentage (14%) of familial occurrence of NP was reported earlier by Greisner and Settipane ( 6) in a smaller group (n=50) of adult patients with NP. Thus, these results strongly suggest the existence of a hereditary factor in the pathogenesis of NP. However, studies of monozygotic twins have not shown that both siblings always develop polyps, indicating that environmental factors are likely to influence the occurrence of NP ( 7, 8). NPs have been described in identical twins, but given the prevalence of nasal polyps, it might be expected that there would be more than a rare report of this finding ( 9).
LINKAGE ANALYSIS AND ASSOCIATION STUDIES ASSOCIATED WITH NPS
In the literature, some studies were able to show linkage of certain phenotypes of NP to candidate gene polymorphisms. Karjalainen et al. ( 10) reported that subjects with a single G-to-T polymorphism in exon 5 at +4,845 of the gene encoding IL-1alpha (IL-1A) were found to have lower risk of developing NP as compared to subjects with common G/G genotype. In another study, polymorphism of IL-4 (IL-4/-590 C-T), a potential determinant of IgE mediated allergic disease, was also found to be associated with a protective mechanism against NPs in the Korean populations ( 11). Recently, another asthma-related Argl6gly polymorphism of the beta2-adrenoceptor gene (ADRBeta2) was found to be associated with an increased risk of NP ( 12). A number of genetic association studies found a significant correlation between certain human leukocyte antigen (HLA) alleles and NP. HLA is the general name of a group of genes in the human major histocompatibility complex (MHC) region on the human chromosome 6 that encodes the cell-surface antigen-presenting proteins. Luxenberger et al. ( 13) reported an association between HLA-A74 and NPs, whereas Molnar-Gabor et al. ( 14) reported that subjects carrying HLA-DR7-DQA1*0201 and HLA-DR7-DQB1*0202 haplotype had a 2 to 3 times odds ratio of developing NP. The risk of developing NP can be as high as 5.53 times in subjects with HLA-DQA1*0201-DQB1*0201 haplotype ( 15). Although several HLA alleles were found to be associated with NP, such susceptibility can be influenced by ethnicity. In the Mexican Mestizo population, increased frequency of the HLA-DRB1*03 allele and of the HLA-DRB1*04 allele were found in patients with NP as compared to healthy controls ( 16).
MULTIPLE GENE EXPRESSIONS IN NASAL POLYPS
The development and persistence of mucosal inflammation in NPs have been reported to be associated with numerous genes and potential single nucleotide polymorphisms (SNPs). The products of these genes determine various disease processes, such as immune modulation or immuno-pathogenesis, inflammatory cells (e.g., lymphocytes, eosinophils and neutrophils) development, activation, migration and life span, adhesion molecule expression, cytokine synthesis, cell-surface receptor display, and processes governing fibrosis and epithelial remodelling.
Gene profiling technologies have demonstrated considerable power in generation of cell and tissue molecular signatures and identification of disease-associated gene expression changes, and represent a rapid and efficient mean to elucidate alterations in cell signalling or metabolic pathways. DNA microarray technology consists of a matrix with attached sequences that allow simultaneous analysis of expression of panels of human genes. Comparison of profiles of genes expressed in disease versus healthy tissues often highlights the involvement of both expected and unsuspected pathologic pathways.
In the literature, gene expression profiles in NPs have been performed by many studies, including the major repertoire of disease-related susceptibility genes or genotypic markers. With the advance of microassay technique, expression profiles of over 10,000 of known and novel genes can be detected. A recent study showed that in NP tissues, 192 genes were upregulated by at least 2-fold, and 156 genes were downregulated by at least 50% in NP tissues as compared to sphenoid sinuses mucosa ( 17). In another study ( 18), microarray analysis was used to investigate the expression profile of 491 immune-associated genes in NPs. The results showed that 87 genes were differentially expressed in the immune-associated gene profile of nasal polyps, and 15 genes showed differential expression in both NPs and healthy controls (turbinates). In other studies, alterations in expression profiles of susceptible genes may contribute to many putative underlying pathophysiological or pathogenic mechanisms of NPs.
Genes associated with inflammation and immunopathogenesis in NPs
NP is a chronic inflammatory disease of the mucous membranes in the nose and paranasal sinuses. The role of the immune system in the pathogenesis of this disease is still unknown. To date, many studies have reported significant changes in presence of numerous inflammatory cells, mediators, cytokines, chemokines, and other inflammatory and immune modulating components in NPs, indicating that chronic inflammation is an important factor in development of NPs irrespective of the etiology.
Chronic rhinosinusitis with nasal polyps (CRSwNP) is characterized by a T(H)2-skewed eosinophilic inflammation, whereas chronic rhinosinusitis without nasal polyps (CRSsNP) represents a predominant T(H)1 milieu ( 18). In this report, the authors were able to show a significantly lower forkhead box P3 (FOXP3) mRNA and transforming growth factor beta-1 (TGF-beta1) protein expression, but a significantly higher T-bet, GATA-3, IL-5, and IL-13 mRNA expression compared with the healthy controls ( Table 1). This T(H)2-skewed eosinophilic inflammation was shown by a decreased expression of multiple antimicrobial innate immune markers, including toll-like receptor 9, human beta-defensin 2 and surfactant protein A, in human sinonasal epithelial cells from CRSwNP. The authors suggested that the impaired mucosal innate immunity may contribute to microbial colonization (e.g., bacteria, fungi and etc.) and abnormal immune responses associated with CRSwNP ( 20). In another study, an increased expression level of complement component C1q in NPs is suggested to be indicative of an ongoing inflammatory response in the nasal mucosa of these patients ( 21). Innate immune recognition of pathogens by sinonasal epithelial cells may play an important role in the pathogenesis of chronic mucosal inflammation in rhinosinusitis (CRS) ( 22, 23). In Table 1, alterations in expression levels of several genes associated with immunopathogenesis in nasal mucosal or NP tissues are indicatives of an ongoing inflammation and impaired mucosal innate immunity in patients with CRS with or without NPs.
Biological functions of inflammatory cells in NPs
The general histopathological classification of NPs is eosinophil-dominated inflammation (80-90%), which appears to be a hallmark of Caucasian NPs ( 3). This statement is well supported by an increased expression of cytokines, chemokines and molecular markers, which are involved in the proliferation, migration, activation and survival of eosinophils ( Table 2). In the literature, IL-5 and eotaxin are most frequently reported molecular markers in NPs, as they are essential for eosinophil development, activation and survival ( 3). The soluble IL-5Rα expression level was also found to be dramatically higher (up to 1,200 times) than IL-5 concentrations in NP ( 37). In addition, infiltration of other types of inflammatory cells, especially lymphocytes and neutrophils, may also play an important role in the inflammatory process underlying the pathogenesis of NPs (38-40). In Table 2, increased expression levels of growth-related oncogene-alpha (GRO-alpha), stem cell factors (SCF) and C-C chemokine ligand 2 are associated with biological functions of neutrophils, mast cells and macrophages. Therefore, in NP studies, one may not rely solely on limited type of cells and number of molecular markers (e., g., IL-5 and eotaxin) as NP is a heterogeneous disease with complex pathogenic mechanisms involved.
Genes associated with structure modification of epithelium in NPs
Histomorphological characterisation of NP tissue reveals frequent epithelial damage, a thickened membrane, and oedematous to sometimes fibrotic stromal tissue, with a reduced number of vessels and glands, but virtually no neutral structure ( 3). Many genetic studies have resulted in an emphasis on the structure modification of the epithelium in NPs. Table 3 shows the changes in expression level of many important genes, which are associated with proliferations of nasal epithelial and endothelial cells, submucosal glandular cells, regulation of goblet cell mucins, tissue remodelling, angiogenesis, impaired electrolyte and water transport across the epithelial cells, epithelial barrier maintenance and repair, and etc. However, pathogenic mechanisms underlying the changes of these structural gene functions are not clear. There are a number of genes which are involved in epithelial barrier maintenance and repair in the inflammatory state of chronic rhinosinusitis (CRS) with NPs. For example, carbonic anhydrase (CA) is a zinc metalloenzyme that participate in the biological processes of various fluid transporting epithelia, including ion and water transport. In this study, a decreased expression level of CA was found to be associated with impaired electrolyte and water transport across the epithelial cell, which will result in oedema of NP tissue ( 56). In another study, the level of epidermal growth factor receptor (EGFR) mRNA in human sinus mucosa was found to be statistically significantly increased compared with that in the healthy controls ( 57). It is therefore suggested by the authors that up-regulation of the EGFR cascade may have an important role regarding mucus production in the sinus mucosa of patients with CRS and CRS with NP associated with hyperplasia and metaplasia of epithelial goblet cells. Epithelial differential and tissue remodelling is also reported to be involved in the development and pathogenic characterization of NPs. It was reported that an increased expression level of PDGF-B, TGF-beta1, and lower expressions of lumican and biglycan were found in NPs as compared to the healthy controls ( Table 3). However, controversial results of TGF-beta1 expression level were also reported by other studies, where the expression levels of TGF-beta1 in NPs appeared to be low in NPs ( Table 3).
Significant genes with other or unclear biological functions in NPs
Using a genome-wide expression microarray, some studies have reported a few genes with significant changes in expression profiles in NP tissue as compared to healthy controls ( Table 4). However, the biological functions of some of these genes (e.g., plasma membrane intrinsic protein [PIP], deleted in malignant brain tumors 1 [DMBT1], Mammaglobin and CC10) underlying the pathogenic mechanisms of NP development are unclear and need to be investigated in future. Mutation and differential expression of the ras family genes contributing to tumourigenesis either through the accumulation of mutations or by aberrant expression in a wide range of human cancers has been well demonstrated. However, a recent study found that K- and H-ras expression levels were elevated, whereas N-ras mRNA levels were lower in NPs and adjacent turbinates as compared to the healthy control tissues. K-ras mRNA levels were up-regulated in advanced-stage polyps, while N-ras levels were found elevated in small polyps ( 78). These findings suggest a potential key role for activated members of ras family genes in terms of their contribution to the development of NPs as well as to the hypertrophy of adjacent turbinates. The presence of aspiring-intolerance in a patient with CRS with or without NPs is associated with a particularly persistent and treatment-resistant form of disease, coexisting usually with severe asthma and referred to as the "aspiring triad" ( 3). In a recent study, genome-wide expression microarray study was performed in 57 patients with three distinct phenotypes: 1) patients with chronic rhinosinusitis and sinonasal polyposis without a history of aspirin allergy (CRS group); 2) patients with chronic rhinosinusitis and sinonasal polyposis with a history of asthma and aspirin allergy (ASA group); and 3) patients with no history or clinical evidence of sinusitis, asthma, or aspirin allergy (control group) ( 79). This study was able to identify five genes (POSTN, MET, PIP, AZGP1, and PPP1R9B) that are likely to play a role in the pathogenesis of sinonasal polyps associated with CRS and ASA ( Table 4).
CONCLUSION
The expression of gene products is regulated at multiple levels, such as during transcription, mRNA processing, translation, phosphorylation and degradation. Although some studies were able to show certain NP associated polymorphisms and genotypes, the present data is still fragmented. Same as for many common human diseases, inherited genetic variation appears to be critical but yet still largely unexplained. Future studies are needed to identify the key genes underlying the development or formation of NP and to investigate the interactions between genetic and environmental factors that influence the complex traits of this disease. Identifying the causal genes and variants in NP is important to the path towards improved prevention, diagnosis and treatment of NPs.
CONFLICT OF INTEREST
Disclosure of potential conflict of interest: the authors declared that no conflict of interest exists.
References
1. Bateman ND, Fahy C, Woolford TJ. Nasal polyps: still more questions than answers. J Laryngol Otol. 2003 1;117(1):1-9. PMID: 12590849.   2. Triglia JM, Nicollas R. Nasal and sinus polyposis in children. Laryngoscope. 1997 7;107(7):963-966. PMID: 9217140.   3. Fokkens W, Lund V, Mullol J. European Position Paper on Rhinosinusitis and Nasal Polyps group. European position paper on rhinosinusitis and nasal polyps 2007. Rhinol Suppl. 2007;(20):1-136. PMID: 17844873.  4. Hellquist HB. Nasal polyps update. Histopathology. Allergy Asthma Proc. 1996;Sep–Oct;17(5):237-242. PMID: 8922142.   5. Rugina M, Serrano E, Klossek JM, Crampette L, Stoll D, Bebear JP, et al. Epidemiological and clinical aspects of nasal polyposis in France: the ORLI group experience. Rhinology. 2002 6;40(2):75-79. PMID: 12091997.  6. Greisner WA 3rd, Settipane GA. Hereditary factor for nasal polyps. Allergy Asthma Proc. 1996;Sep–Oct;17(5):283-286. PMID: 8922148.   7. Lockey RF, Rucknagel DL, Vanselow NA. Familial occurrence of asthma, nasal polyps and aspirin intolerance. Ann Intern Med. 1973 1;78(1):57-63. PMID: 4682309.   8. Settipane GA. Asthma, aspirin intolerance and nasal polyps. N Engl Reg Allergy Proc. 1986;Jan–Feb;7(1):32-37. PMID: 3475535.   9. Drake-Lee A. Nasal polyps in identical twins. J Laryngol Otol. 1992 12;106(12):1084-1085. PMID: 1487667.   10. Karjalainen J, Joki-Erkkila VP, Hulkkonen J, Pessi T, Nieminen MM, Aromaa A, et al. The IL1A genotype is associated with nasal polyposis in asthmatic adults. Allergy. 2003 5;58(5):393-396. PMID: 12752325.   11. Yea SS, Yang YI, Park SK, Jang WH, Lee SS, Seog DH, et al. Interleukin-4 C-590T polymorphism is associated with protection against nasal polyps in a Korean population. Am J Rhinol. 2006;Sep–Oct;20(5):550-553. PMID: 17063753.   12. Bussu F, Tiziano FD, Giorgio A, Pinto AM, De Corso E, Angelozzi C, et al. Argl6gly polymorphism of the beta2-adrenoceptor gene (ADRBeta2) as a susceptibility factor for nasal polyposis. Am J Rhinol. 2007;May–Jun;21(3):378-382. PMID: 17621827.   13. Luxenberger W, Posch U, Berghold A, Hofmann T, Lang-Loidolt D. HLA patterns in patients with nasal polyposis. Eur Arch Otorhinolaryngol. 2000;257(3):137-139. PMID: 10839486.   14. Molnar-Gabor E, Endreffy E, Rozsasi A. HLA-DRB1, -DQA1, and -DQB1 genotypes in patients with nasal polyposis. Laryngoscope. 2000 3;110(3 Pt 1):422-425. PMID: 10718431.   15. Fajardo-Dolci G, Solorio-Abreu J, Romero-Alvarez JC, Zavaleta-Villa B, Cerezo-Camacho O, Jimenez-Lucio R, et al. DQA1 and DQB1 association and nasal polyposis. Otolaryngol Head Neck Surg. 2006 8;135(2):243-247. PMID: 16890076.   16. Ramirez-Anguiano J, Yamamoto-Furusho JK, Barquera R, Beltran O, Granados J. Association of HLA-DR3 and HLA-DR4 with sinonasal polyposis in Mexican Mestizos. Otolaryngol Head Neck Surg. 2006 7;135(1):90-93. PMID: 16815190.   17. Liu Z, Kim J, Sypek JP, Wang IM, Horton H, Oppenheim FG, et al. Gene expression profiles in human nasal polyp tissues studied by means of DNA microarray. J Allergy Clin Immunol. 2004 10;114(4):783-790. PMID: 15480316.   18. Wang X, Dong Z, Zhu DD, Guan B. Expression profile of immune-associated genes in nasal polyps. Ann Otol Rhinol Laryngol. 2006 6;115(6):450-456. PMID: 16805377.   19. Van Bruaene N, Perez-Novo CA, Basinski TM, Van Zele T, Holtappels G, De Ruyck N, et al. T-cell regulation in chronic paranasal sinus disease. J Allergy Clin Immunol. 2008 6;121(6):1435-1441. PMID: 18423831.   20. Ramanathan M Jr, Lee WK, Spannhake EW, Lane AP. Th2 cytokines associated with chronic rhinosinusitis with polyps down-regulate the antimicrobial immune function of human sinonasal epithelial cells. Am J Rhinol. 2008;Mar–Apr;22(2):115-121. PMID: 18416964.    21. Baruah P, Trimarchi M, Dumitriu IE, Dellantonio G, Doglioni C, Rovere-Querini P, et al. Innate responses to Aspergillus: role of C1q and pentraxin 3 in nasal polyposis. Am J Rhinol. 2007;Mar–Apr;21(2):224-230. PMID: 17424885.   22. Ramanathan M Jr, Lee WK, Dubin MG, Lin S, Spannhake EW, Lane AP. Sinonasal epithelial cell expression of toll-like receptor 9 is decreased in chronic rhinosinusitis with polyps. Am J Rhinol. 2007;Jan–Feb;21(1):110-116. PMID: 17283572.   23. Figueiredo CR, Silva ID, Weckx LL. Inflammatory genes in nasal polyposis. Curr Opin Otolaryngol Head Neck Surg. 2008 2;16(1):18-21. PMID: 18197016.   24. Barekzi E, Roman J, Hise K, Georas S, Steinke JW. Lysophosphatidic acid stimulates inflammatory cascade in airway epithelial cells. Prostaglandins Leukot Essent Fatty Acids. 2006 6;74(6):357-363. PMID: 16725318.   25. Chen PH, Fang SY. The expression of human antimicrobial peptide LL-37 in the human nasal mucosa. Am J Rhinol. 2004;Nov–Dec;18(6):381-385. PMID: 15706986.   26. Cheng YK, Hwang GY, Lin CD, Tsai MH, Tsai SW, Chang WC. Altered expression profile of superoxide dismutase isoforms in nasal polyps from nonallergic patients. Laryngoscope. 2006 3;116(3):417-422. PMID: 16540901.   27. Chen PH, Fang SY. Expression of human beta-defensin 2 in human nasal mucosa. Eur Arch Otorhinolaryngol. 2004 5;261(5):238-241. PMID: 14504864.   28. Pujols L, Mullol J, Alobid I, Roca-Ferrer J, Xaubet A, Picado C. Dynamics of COX-2 in nasal mucosa and nasal polyps from aspirin-tolerant and aspirin-intolerant patients with asthma. J Allergy Clin Immunol. 2004 10;114(4):814-819. PMID: 15480320.   29. Ohkawara Y, Lim KG, Xing Z, Glibetic M, Nakano K, Dolovich J, et al. CD40 expression by human peripheral blood eosinophils. J Clin Invest. 1996 4 01; 97(7):1761-1766. PMID: 8601642.    30. Lee CH, Rhee CS, Min YG. Cytokine gene expression in nasal polyps. Ann Otol Rhinol Laryngol. 1998 8;107(8):665-670. PMID: 9716868.   31. Mullol J, Xaubet A, Gaya A, Roca-Ferrer J, Lopez E, Fernandez JC, et al. Cytokine gene expression and release from epithelial cells. A comparison study between healthy nasal mucosa and nasal polyps. Clin Exp Allergy. 1995;25(7):607-615. PMID: 8521179.   32. Finotto S, Ohno I, Marshall JS, Gauldie J, Denburg JA, Dolovich J, et al. TNF-alpha production by eosinophils in upper airways inflammation (nasal polyposis). J Immunol. 1994;153(5):2278-2289. PMID: 8051424.    33. Picado C, Fernandez-Morata JC, Juan M, Roca-Ferrer J, Fuentes M, Xaubet A, et al. Cyclooxygenase-2 mRNA is downexpressed in nasal polyps from aspirin-sensitive asthmatics. Am J Respir Crit Care Med. 1999;160(1):291-296. PMID: 10390414.   34. Yamada T, Fujieda S, Yanagi S, Yamamura H, Inatome R, Yamamoto H, et al. IL-1 induced chemokine production through the association of Syk with TNF receptor-associated factor-6 in nasal fibroblast lines. J Immunol. 2001;167(1):283-288. PMID: 11418661.   35. Yamada T, Fujieda S, Yanagi S, Yamamura H, Inatome R, Sunaga H, et al. Protein-tyrosine kinase Syk expressed in human nasal fibroblasts and its effect on RANTES production. J Immunol. 2001;166(1):538-543. PMID: 11123334.   36. Zaravinos A, Bizakis J, Spandidos DA. RKIP and BRAF aberrations in human nasal polyps and the adjacent turbinate mucosae. Cancer Lett. 2008 6 18; 264(2):288-298. PMID: 18329792.   37. Gevaert P, Bachert C, Holtappels G, Novo CP, Van der Heyden J, Fransen L, et al. Enhanced soluble interleukin-5 receptor alpha expression in nasal polyposis. Allergy. 2003 5;58(5):371-379. PMID: 12752323.   38. Hao J, Pang YT, Wang DY. Diffuse mucosal inflammation in nasal polyps and adjacent middle turbinate. Otolaryngol Head Neck Surg. 2006 2;134(2):267-275. PMID: 16455376.   39. Jareoncharsri P, Bunnag C, Tunsuriyawrong P, Assasin P, Muangsomboon S. Clinical and histopathological classification of nasal polyps in Thais. Siriraj Hosp Gaz. 2002;54:689-697.
40. Zhang N, Holtappels G, Claeys C, Huang G, van Cauwenberge P, Bachert C. Pattern of inflammation and impact of Staphylococcus aureus enterotoxins in nasal polyps from southern China. Am J Rhinol. 2006;Jul–Aug;20(4):445-450. PMID: 16955777.  41. Meyer JE, Bartels J, Gorogh T, Sticherling M, Rudack C, Ross DA, et al. The role of RANTES in nasal polyposis. Am J Rhinol. 2005;Jan–Feb;19(1):15-20. PMID: 15794069.   42. Bartels J, Maune S, Meyer JE, Kulke R, Schluter C, Rowert J, et al. Increased eotaxin-mRNA expression in non-atopic and atopic nasal polyps: comparison to RANTES and MCP-3 expression. Rhinology. 1997 12;35(4):171-174. PMID: 9532637.  43. Davidsson A, Danielsen A, Viale G, Olofsson J, Dell'Orto P, Pellegrini C, et al. Positive identification in situ of mRNA expression of IL-6, and IL-12, and the chemotactic cytokine RANTES in patients with chronic sinusitis and polypoid disease. Clinical relevance and relation to allergy. Acta Otolaryngol. 1996 7;116(4):604-610. PMID: 8831850.   44. Chen YS, Langhammer T, Westhofen M, Lorenzen J. Relationship between matrix metalloproteinases MMP-2, MMP-9, tissue inhibitor of matrix metalloproteinases-1 and IL-5, IL-8 in nasal polyps. Allergy. 2007 1;62(1):66-72. PMID: 17156344.   45. Choi BR, Kwon JH, Gong SJ, Kwon MS, Cho JH, Kim JH, et al. Expression of glucocorticoid receptor mRNAs in glucocorticoid-resistant nasal polyps. Exp Mol Med. 2006 10 31; 38(5):466-473. PMID: 17079862.   46. Yue J, Chen J, Kong W, Tan H, Shu H, Shi Q. The concentration and expression of IL-4, IL-5, IL-6, IL-8 in human nasal polyps tissues. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 2006 6;20(11):484-486. PMID: 16929824.  47. Guan GM, Dong Z, Lu M. Expression of aquaporin-1 and Bcl-2 mRNA in eosinophils of the nasal polyps and its significance. Zhonghua Er Bi Yan Hou Ke Za Zhi. 2004 8;39(8):476-478. PMID: 15563082.  48. Li M, Dong Z, Yang Z, Bai Y. Protein kinase C in proliferation and infiltration of eosinophils in nasal polyp. Chin Med J (Engl). 2003 10;116(10):1553-1556. PMID: 14570622.  49. Molinaro RJ, Bernstein JM, Koury ST. Localization and quantitation of eotaxin mRNA in human nasal polyps. Immunol Invest. 2003 8;32(3):143-154. PMID: 12916705.   50. Shin SH, Park JY, Jeon CH, Choi JK, Lee SH. Quantitative analysis of eotaxin and RANTES messenger RNA in nasal polyps: association of tissue and nasal eosinophils. Laryngoscope. 2000 8;110(8):1353-1357. PMID: 10942140.   51. Cardell LO, Bogefors J, Bjartell A, Adner M, Uddman R, Egesten A. Topical steroids do not downregulate expression of growth-related oncogene-alpha in nasal polyps. Acta Otolaryngol. 2006 4;126(4):375-380. PMID: 16608789.   52. Kim YK, Nakagawa N, Nakano K, Sulakvelidze I, Dolovich J, Denburg J. Stem cell factor in nasal polyposis and allergic rhinitis: increased expression by structural cells is suppressed by in vivo topical corticosteroids. J Allergy Clin Immunol. 1997 9;100(3):389-399. PMID: 9314353.   53. Shun CT, Lin SK, Hong CY, Kok SH, Juan YH, Wang CC, et al. C-C chemokine ligand 2 gene expression in nasal polyp fibroblasts: possible implication in the pathogenesis of nasal polyposis. Ann Otol Rhinol Laryngol. 2005 11;114(11):879-885. PMID: 16358608.   54. Liu B, Wu J, Fan J, Peng Y. Gene expression profiles in human nasal polyps studied by DNA microarray. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2008 6;22(11):495-497. PMID: 18727517.  55. Qiu ZF, Han DM, Zhang L, Zhang W, Fan EZ, Cui SJ, et al. Expression of survivin and enhanced polypogenesis in nasal polyps. Am J Rhinol. 2008;Mar–Apr;22(2):106-110. PMID: 18336724.   56. Kim TH, Lee HM, Lee SH, Kim HK, Lee JH, Oh KH. Down-regulation of carbonic anhydrase isoenzymes in nasal polyps. Laryngoscope. 2008 10;118(10):1856-1861. PMID: 18622311.   57. Ding GQ, Zheng CQ, Bagga SS. Up-regulation of the mucosal epidermal growth factor receptor gene in chronic rhinosinusitis and nasal polyposis. Arch Otolaryngol Head Neck Surg. 2007 11;133(11):1097-1103. PMID: 18025312.   58. Rho HS, Lee SH, Lee HM, Jung HH, Choi J, Park MK, et al. Overexpression of hepatocyte growth factor and its receptor c-Met in nasal polyps. Arch Otolaryngol Head Neck Surg. 2006 9;132(9):985-989. PMID: 16982975.   59. Dong Z, Guan G, Chang W. Expression of cell proliferation and apoptosis gene associated protein on nasal polyps and its significance. Zhonghua Er Bi Yan Hou Ke Za Zhi. 2000 12;35(6):429-431. PMID: 12768752.  60. Powers MR, Qu Z, LaGesse PC, Liebler JM, Wall MA, Rosenbaum JT. Expression of basic fibroblast growth factor in nasal polyps. Ann Otol Rhinol Laryngol. 1998 10;107(10 Pt 1):891-897. PMID: 9794621.   61. Ishibashi T, Tanaka T, Nibu K, Ishimoto S, Kaga K. Keratinocyte growth factor and its receptor messenger RNA expression in nasal mucosa and nasal polyps. Ann Otol Rhinol Laryngol. 1998 10;107(10 Pt 1):885-890. PMID: 9794620.   62. Ingle RR, Setzen G, Koltai PJ, Monte D, Pastore J, Jennings TA. p53 protein expression in benign lesions of the upper respiratory tract. Arch Otolaryngol Head Neck Surg. 1997 3;123(3):297-300. PMID: 9076236.   63. Martinez-Anton A, Debolos C, Garrido M, Roca-Ferrer J, Barranco C, Alobid I, et al. Mucin genes have different expression patterns in healthy and diseased upper airway mucosa. Clin Exp Allergy. 2006 4;36(4):448-457. PMID: 16630149.   64. Ali MS, Wilson JA, Bennett M, Pearson JP. Mucin gene expression in nasal polyps. Acta Otolaryngol. 2005 6;125(6):618-624. PMID: 16076710.   65. Young Kim J, Kim CH, Kim KS, Choi YS, Lee JG, Yoon JH. Extracellular signal-regulated kinase is involved in tumor necrosis factor-alpha-induced MUC5AC gene expression in cultured human nasal polyp epithelial cells. Acta Otolaryngol. 2004 10;124(8):953-957. PMID: 15513533.   66. Kim CH, Song KS, Kim SS, Kim HU, Seong JK, Yoon JH. Expression of MUC5AC mRNA in the goblet cells of human nasal mucosa. Laryngoscope. 2000 12;110(12):2110-2113. PMID: 11129031.   67. Bai CH, Song SY, Kim YD. Effect of glucocorticoid on the MUC4 gene in nasal polyps. Laryngoscope. 2007 12;117(12):2169-2173. PMID: 17891050.   68. Ding GQ, Zheng CQ. The expression of MUC5AC and MUC5B mucin genes in the mucosa of chronic rhinosinusitis and nasal polyposis. Am J Rhinol. 2007;May–Jun;21(3):359-366. PMID: 17621824.   69. Burgel PR, Escudier E, Coste A, Dao-Pick T, Ueki IF, Takeyama K, et al. Relation of epidermal growth factor receptor expression to goblet cell hyperplasia in nasal polyps. J Allergy Clin Immunol. 2000 10;106(4):705-712. PMID: 11031341.   70. Fang SY, Yang BC. Overexpression of Fas-ligand in human nasal polyps. Ann Otol Rhinol Laryngol. 2000 3;109(3):267-270. PMID: 10737309.   71. Ito A, Hirota S, Mizuno H, Kawasaki Y, Takemura T, Nishiura T, et al. Expression of vascular permeability factor (VPF/VEGF) messenger RNA by plasma cells: possible involvement in the development of edema in chronic inflammation. Pathol Int. 1995 10;45(10):715-720. PMID: 8563931.   72. Ohno I, Nitta Y, Yamauchi K, Hoshi H, Honma M, Woolley K, et al. Eosinophils as a potential source of platelet-derived growth factor B-chain (PDGF-B) in nasal polyposis and bronchial asthma. Am J Respir Cell Mol Biol. 1995 12;13(6):639-647. PMID: 7576701.   73. Zaravinos A, Soufla G, Bizakis J, Spandidos DA. Expression analysis of VEGFA, FGF2, TGFbeta1, EGF and IGF1 in human nasal polyposis. Oncol Rep. 2008 2;19(2):385-391. PMID: 18202785.  74. Lee SH, Park JH, Oh BH, Jung KY, Lee HM, Choi JO, et al. Analysis of proteoglycan gene messages in human nasal mucosa and nasal polyp using dot blot hybridization. Acta Otolaryngol. 2001 4;121(3):398-402. PMID: 11425208.   75. Richer SL, Truong-Tran AQ, Conley DB, Carter R, Vermylen D, Grammer LC, et al. Epithelial genes in chronic rhinosinusitis with and without nasal polyps. Am J Rhinol. 2008;May–Jun;22(3):228-234. PMID: 18588753.    76. Rostkowska-Nadolska B, Kapral M, Mazurek U, Gawron W, Pres K. Co-expression of the TGF-beta1 and TGF-beta2 isoforms in nasal polyps and in healthy mucosa. Postepy Hig Med Dosw (Online). 2007;61:702-707. PMID: 18059253.  77. Figueiredo CR, Santos RP, Silva ID, Weckx LL. Microarray cDNA to identify inflammatory genes in nasal polyposis. Am J Rhinol. 2007;Mar–Apr;21(2):231-235. PMID: 17424886.   78. Zaravinos A, Bizakis J, Soufla G, Sourvinos G, Spandidos DA. Mutations and differential expression of the ras family genes in human nasal polyposis. Int J Oncol. 2007 11;31(5):1051-1059. PMID: 17912430.  79. Stankovic KM, Goldsztein H, Reh DD, Platt MP, Metson R. Gene expression profiling of nasal polyps associated with chronic sinusitis and aspirin-sensitive asthma. Laryngoscope. 2008 5;118(5):881-889. PMID: 18391768.   80. Fritz SB, Terrell JE, Conner ER, Kukowska-Latallo JF, Baker JR. Nasal mucosal gene expression in patients with allergic rhinitis with and without nasal polyps. J Allergy Clin Immunol. 2003 12;112(6):1057-1063. PMID: 14657858.   81. Watanabe S, Suzaki H. Changes of glucocorticoid receptor expression in the nasal polyps of patients with chronic sinusitis following treatment with glucocorticoid. In Vivo. 2008;22(1):37-42. PMID: 18396779. 
Table 1
Expression profile of genes associated with inflammation and immunopathogenesis in nasal polyposis 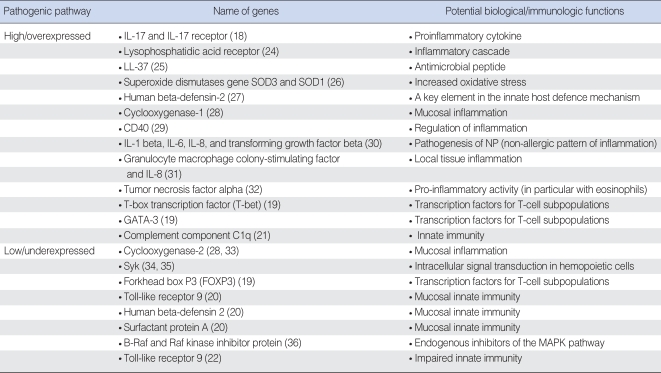
Table 2
Expression profile of genes associated with the biological functions of inflammatory cells in nasal polyposis 
Table 3
Expression profile of genes associated with structure modification of epithelium in nasal polyposis 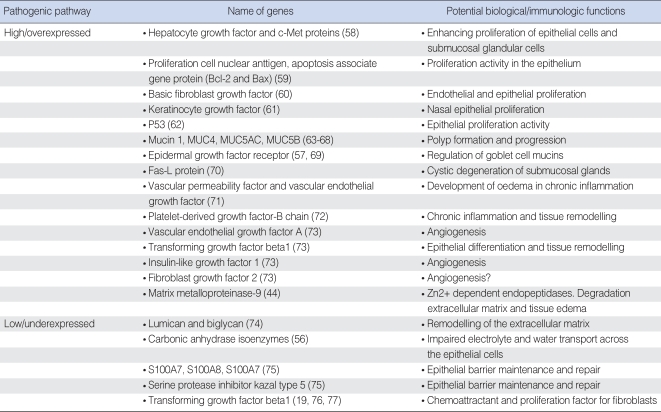
Table 4
Significant genes with other or unclear biological functions in nasal polyposis 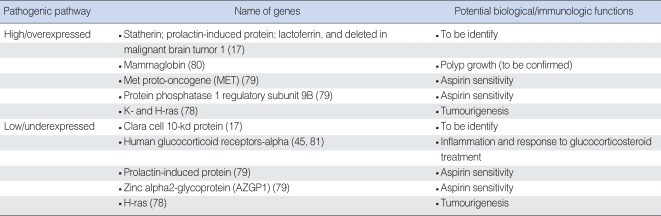
|
|













