INTRODUCTION
Fungal infection of the nasal cavity and paranasal sinuses has been an uncommon disease since Mackenzie first reported it in 1893 [1]; however, its incidence is gradually increasing. The unilateral maxillary fungal ball (FB) is defined as a chronic infection within the lumen of a sinus cavity, usually in the maxillary antrum, characterized by histological evidence of fungal hyphae and no microscopic evidence of fungal invasion, and occurring in immunocompetent hosts [1,2].
The pathogenesis of FB is still unclear. Stammberger [3] hypothesized that the pathogenesis of fungal sinusitis began with nourishment of the fungus by purulent secretions from bacterial and viral superinfection, which was followed by the growth of fungal hyphae in a low-pH environment provided by the stenosed ostiomeatal complex (OMC). Eloy et al. [4] suggested that sinus hypoventilation secondary to ostial dyspermeability plays an important role in trapping fungal spores and providing anaerobic conditions for the development of FB. Recently, Tsai et al. [5] proposed some criticism of this hypothesis. By analyzing the Lund-McKay scores in paranasal sinus computed tomography (CT) scans, they demonstrated that obstruction of the OMC was not clearly demonstrable in the onset of an FB.
Deviation of the nasal septum to one side of the nose causes chronic changes in nasal airflow, characterized by an ipsilateral reduction in nasal airflow and a contralateral increase in nasal airflow. Septal deviation has also been reported to be a cause of chronic sinusitis because of its effect on the structure of the OMC and the obstruction of normal mucociliary clearance from the frontal, maxillary, and anterior ethmoidal sinuses [6]. The middle meatus is an air space lateral to the middle turbinate and medial to the uncinate process and ethmoid bulla [7], which the frontal, maxillary, and anterior ethmoidal sinuses drains into [8]. In most cases of invasive fungal disease, it is known that the middle turbinate and meatus are important structures to allow fungus to seed and initiate the invasive process because these structures are exposed to the greatest volume of nasal airflow and the mucosal disruption, damage to the mucociliary system, and changes in the normal mucosal flora are common at the middle turbinate and meatus secondary to bacterial sinusitis, allergy, drying, and mechanical damage [9,10]. However, little has been reported regarding the relationships between the FB, septal deviation, and intranasal airflow. If the pathogenesis of the FB originates from specific anatomic variations such as septal deviation and intranasal airflow, the anatomic variations would be significantly associated with localization of FB in patients with FB or the anatomic measurement in patients with FB would be significantly different from those in patients with chronic sinusitis or no sinus disease. In this study, we evaluated the CT scans for the septal deviation and used a three-dimensional (3D) reconstruction technique to make more exact volumetric analyses. We aimed to verify the hypothesis that the septal deviation and intranasal volume could play a significant pathogenic role in patients with FB.
MATERIALS AND METHODS
This study was approved by Institutional Review Board of the Catholic Medical Center Clinical Research Coordinating Center (HC11RISE0051). Records from patients who had undergone diagnostic paranasal high resolution CT (HRCT) scans at our clinic between January 2004 and December 2010 were examined retrospectively for this study. Three groups of patients were included; the first group was fifty-six patients who were confirmed FB with histopathological sections. The second group and third group were selected randomly. The second group was composed of fifty-six patients who fulfilled diagnostic criteria for unilateral chronic rhinosinusitis (CRS) and had failed to respond to medical management. Patients having CRS had to have two or more recognized symptoms for over twelve weeks. These symptoms included nasal obstruction, congestion or nasal discharge (with anterior nasal or postnasal drip), facial pain or pressure, or reduction of the sense of smell. The third group was twenty eight patients who visited at our clinic for evaluating the other symptoms except the nasal symptom and showed the non-specific finding of the paranasal sinus in CT scan. Patients who had the opacification or mucosal thickening of OMC and the expanded middle meatus volume caused by fungus infection or chronic inflammation were excluded under reviewing CT scans. Patients were also excluded if they had a history of facial trauma, septal or sinus surgery, or endodontic treatment on the maxillary teeth that could influence the airflow pathway or sinus mucosa. No significant age difference existed among three groups (P>0.05).
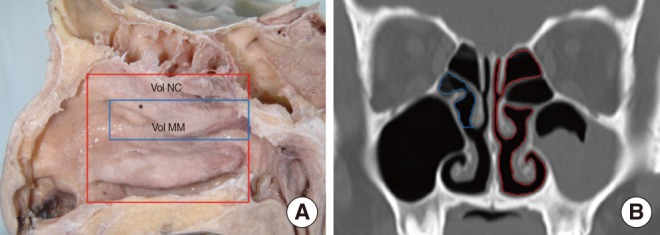
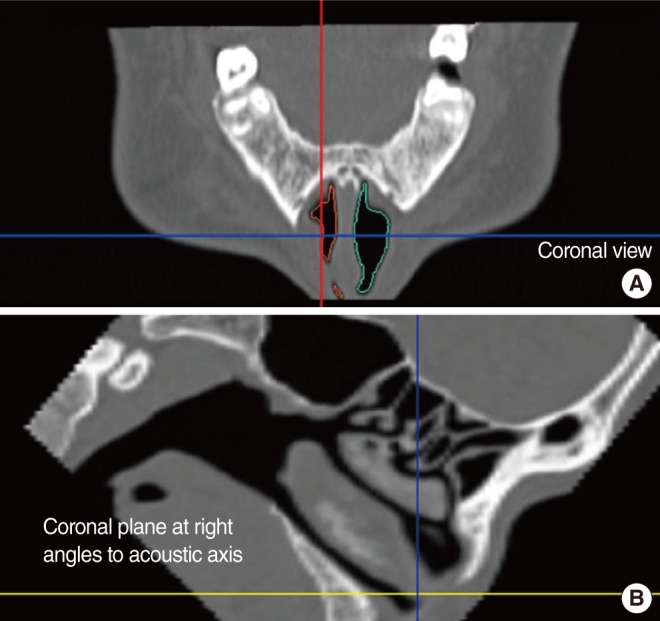
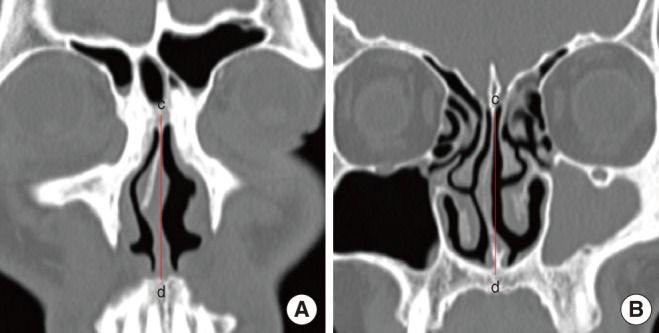
HRCT scans were performed in 2-mm slices with a CT scanner (Sensation 16; Siemens Medical Systems, Munich, Germany). After the imaging data were stored in a Digital Imaging and Communication in Medicine (DICOM) file, they were imported to a personal computer. The coronal views were examined by one observer, layer by layer, using 3D-DOCTOR (Able Software Co., Lexington, MA, USA). The software provided line segmentation. We marked several points according to anatomic boundaries, and the software drew straight lines between each set of two adjacent points. A 3D reconstruction was generated after segmentation. Volumetric boundaries for imaginary air spaces were set up using the septum, each turbinate, and the floor and roof of the nasal cavity. The volume of the nasal cavity was defined as the volume of the imaginary air space limited coronally from the anterior end of the inferior turbinate to its posterior end, sagittally from the lateral nasal wall to the septum, and axially from the roof of the nasal cavity to its floor. The volume of the middle meatus of the nasal cavity was defined as the imaginary air space limited by the lateral surface and the anterior, posterior, and inferior ends of the middle turbinate and the lateral wall of the nasal cavity (Fig. 1). According to each definition, each space was reconstructed as a 3D image from HRCT scans, and the volume was automatically calculated with the software (Fig. 2). The nasal valve area was measured by reformatted cross-sectional images orthogonal to the imaginary air pathway and the angle between the hard palate and the plane of nasal valve area, which was nearly 50°, was selected for the coronal reconstructed images (Fig. 3) [11].
Nasal septal deviation was divided into anterior deviation (deviation at the anterior end of the inferior turbinate) and posterior deviation (deviation at the OMC level, which showed the frontal sinus opening and maxillary sinus opening on the coronal view) to identify whether the nasal septal deviation has an effect on the nasal valve area or on the OMC, and it was assessed on the CT scans (Fig. 4) [12,13].
Statistical analysis was performed using SPSS ver. 18 (SPSS Inc., Chicago, IL, USA). Pearson's χ2 test was applied to define the relationship of the FB with anatomic measurements and compare the difference between the FB and CRS in lesion-side middle meatus volumes. The statistical significance of the volumetric and areal differences between groups was determined by one-way analysis of variance (ANOVA). A P-value of less than 0.05 was considered significant.
RESULTS
Of the 56 patients with FB, 41 (73%) had nasal septal deviation; 7 (17%) had a convexity on only the anterior portion, 6 (15%) had a convexity on only the posterior portion, and 28 (68%) had convexities on both the anterior and posterior portions. Three patients had a biconvex deviation, which was a deviation in different directions on the anterior and posterior portions. Twenty-eight (50%) had smaller lesion-side nasal cavity volumes, and 28 (50%) had smaller lesion-side nasal valve areas. No statistically significant association was found between septal deviation and ipsilateral FB, between the volume of the nasal cavity and FB (P>0.05), or between the nasal valve area and FB (P>0.05).
The middle meatus volume was measured in 56 patients with FB; 46 (82%) had larger lesion-side middle meatus volumes. Of the 56 patients with CRS, the middle meatus volume was measured and 48 (86%) had smaller lesion-side middle meatus volumes. There was a statistically positive association between the relatively larger volume of the middle meatus and FB and between the relatively lesser volume of the middle meatus and CRS. In addition, comparing FB and CRS in view of the relationship between the volume of middle meatus and the lesion-side of the disease, there was a statistically significant difference between two diseases (P<0.05).
Of three groups, mean nasal cavity volume, mean middle meatus volume, and mean nasal valve area in both sides were evaluated. There was no significant mean volumetric and areal difference among the lesion-side and contralateral-side in groups with FB and CRS and both side in group with no sinus disease (P>0.05) (Table 1).
DISCUSSION
These results did not support the hypothesis that anatomic variations could play a significant pathogenic role in patients with FB, which would suggest that other immune diseases or factors affecting the mucus may be more important than anatomical variations. However, we showed that the relatively largeness of the middle meatus volume was associated with the localization of the FB in FB group despite identifying no significant difference and cut-off values of middle meatus volume. It meant that the FB might prefer one maxillary sinus with larger middle meatus in patients who would have other risk factors of FB.
In previous study [5], by comparing the Lund-McKay scores for the anterior ethmoidal and frontal sinuses of two groups of patients suffering from CRS and FB, it was demonstrated that obstruction of the OMC played a relevant role in the onset of rhinosinusitis, whereas a similar finding was not clearly demonstrable in the onset of an FB. Therefore, it was suggested that FB was not associated with OMC obstruction and that another factors would be responsible. We speculated that other anatomic factors such as septal deviation, nasal valve area, or nasal airflow volume would be associated with the pathogenesis of FB.
A paranasal sinus CT scan, performed in the coronal plane, gives an excellent view of the nasal passages [14]. Recently, the 3D reconstructions of CT scans were used in order to evaluate airflow volume and olfactory function [15]. For the identification of septal deviation and measurements of airflow volume of nasal cavity and middle meatus, paranasal sinus CT scans were reviewed and reconstructed into 3D images. Unlike previous studies which did not consider the position of septal deviation in the nasal cavity [16,17], we classified septal deviation as anterior deviation (in nasal valve) and posterior deviation (in OMC level) because the position of septal deviation could affect nasal resistance, nasal airflow, incidence and severity of sinus disease [18,19]. In FB of our study, 31% of cases with septal deviation had deviation on the only anterior portion or posterior portion and 7% had deviation of different directions on the anterior and posterior portions, which meant that more detailed classification of septal deviation was needed to evaluate the effect of deviation on nasal cavity or sinus diseases.
To evaluate whether the localization of FB would be associated with anatomic factors in FB group, we compared the septal deviation, intranasal volume, and area of nasal valve of FB-ipsilateral sides with those of contralateral sides in FB group. Septal deviation, nasal-cavity volume, and nasal valve area were not associated with the occurrence of FB-ipsilateral sides. However, the relatively larger middle meatus volume was associated with the localization of the FB, whereas relatively lesser middle meatus volume was associated with the lesion-side in CRS group. Additionally, to identify the characteristics of anatomy in FB group, we compared the FB group with CRS group and normal group in view of the volumetric and areal measurements. The volumetric and areal measurements such as nasal cavity, middle meatus, and nasal valve in FB-ipsilateral sides were not significantly different from those in contralateral sides as well as other groups.
There were several possible reasons why the volume of middle meatus except other factors would have positive effect on localization of the FB on the basis of our results. First, the middle meatus bears the major part of the inspiratory nasal airflow [20], and the airflow changes from an upward to a horizontal direction, which causes many inspired particles to impact here. Studies have shown that this area is the primary site for the development of adenocarcinomas in woodworkers and the development of mucosal changes and squamous carcinoma in nickel-exposed workers [21]. Second, it is known that excessive nasal airflow could predispose to nasal disease because abnormal inspiratory airflow cause drying of the nasal epithelium that leads to crusting and infection [6]. Deviation of the nasal septum to one side of the nose would cause chronic changes in nasal airflow, with an ipsilateral reduction in nasal airflow and a contralateral increase in nasal airflow. However, because the nasal cavity volume, nasal septal deviation, and nasal valve area could not influence the middle meatus volume directly, these anatomic factors would have no effect on the mucociliary clearance of the middle meatus and related incidence of FB. On the other hands, an increase in the middle meatus volume could cause an increase in the airflow in the middle meatus and impairment of mucociliary clearance. Third, the protective mucociliary function of the nasal middle meatus is lower than that of the nasal common meatus, because the mucociliary transport rate of the nasal middle meatus is lower than that of the nasal common meatus [22]. Based on this fact, it is possible that the mucociliary function of the middle meatus is more susceptible to an increase in airflow and that the volume of the middle meatus is associated with the incidence of the ipsilateral FB.
This study of the pathogenesis regarding maxillary FBs had two limitations. First, these results could not explain FBs that occurred in the lesser-volume side of the middle meatus. Second, the study was limited by the use of CT measurements to estimate the anatomy of the paranasal sinus, nasal valve area, and volume of the airflow. Coronal reconstructions, software errors, and human errors decrease the accuracy of measurements. However, to our knowledge, this study is the first to describe the relationship between FB and anatomic variations in this manner; the advent of high-resolution computed tomography and advanced imaging guidance software allows for evaluation of structures and volumes in detail. Our results may be used as a reference to approach other factors affecting the incidence of FB.
In conclusion, the investigation of the relationship between FB, nasal septal deviation, and airflow revealed that the volume of the middle meatus had significant association with the localization of FB. We concluded that an increase in the volume of the middle meatus may cause impairment of the mucociliary clearance in the middle meatus, and fungal spores that are not cleared may result in FB. However, other main factors are likely involved in FB pathogenesis, and more research on this issue is required.