|
 |
- Search
AbstractObjectivesThe authors would report the results of definitive radiation therapy (RT) for early glottic cancer by two different radiation dose schedules.
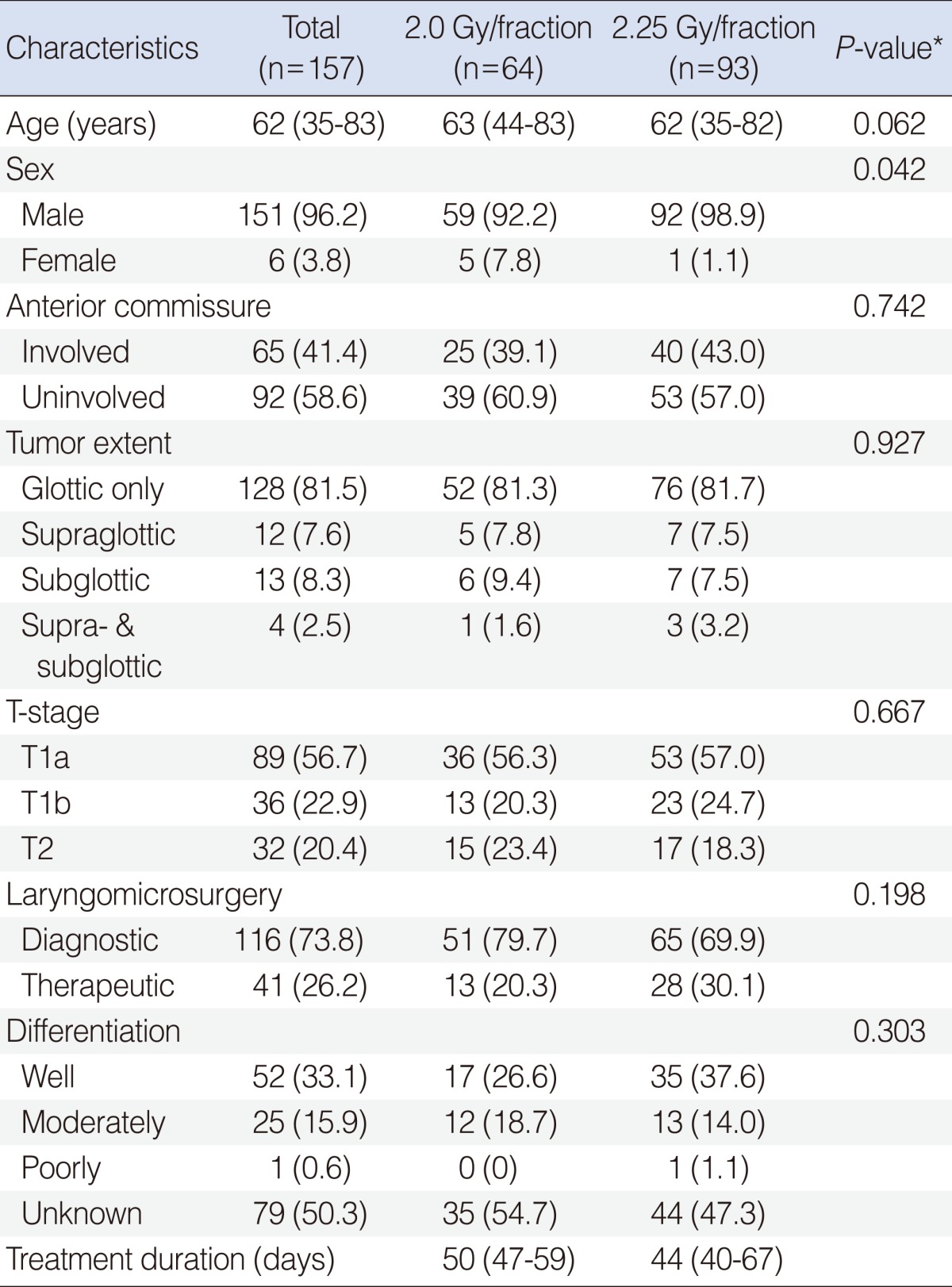
MethodsFrom February of 1995 till June of 2008, 157 patients with T1-2N0 glottic cancer were treated with curative RT at Samsung Medical Center. All patients had squamous cell carcinoma, and there were 89 patients (56.7%) with T1a, 36 (22.9%) with T1b, and 32 (20.4%) with T2. Two different radiation dose schedules were used: 70 Gy in 35 fractions to 64 patients (40.8%, group A); and 67.5 Gy in 30 fractions to 93 patients (59.2%, group B). The median treatment durations were 50 days (range, 44 to 59 days) and 44 days (range, 40 to 67 days) in the groups A and B, respectively.
ResultsThe median follow-up durations were 85 and 45 months for the groups A and B. No severe late complication of RTOG grade 3 or higher was observed, and there was no difference in acute or chronic complication between the groups. Twenty-four patients experienced treatment failure: local recurrence only in 19 patients; regional recurrence only in one; combined local and regional recurrence in four; and systemic metastasis in none. The overall 5-year disease-free survival and disease-specific survival rates were 84.7% and 94.8%. The disease-free survival rate in the group B was better (78.3% vs. 90.8%, P=0.031). This difference was significant only in T1 stage (83.4% vs. 94.6%, P=0.025), but not in T2 (62.7% vs. 60.6%, P=0.965). Univariate analysis showed that the tumor extent, cord mobility, T-stage, and the dose schedule had significant influence on the disease-free survival, and multivariate analysis showed that only the tumor extent and the dose schedule were associated with the disease-free survival.
The glottis is the most commonly involved subsite of laryngeal cancer (1). Early appearing subjective symptoms of voice change and throat discomfort frequently bring the patients to medical attention at early stages of glottic cancer, and the lesions are easily visible by laryngoscopic evaluation, which enables early suspicion and diagnosis. The paucity of the lymphatic capillary beneath the glottic mucosa usually limits the incidence of lymph node metastasis below 5%, if diagnosed early. Based on this underlying pathophysiology, high local control and survival rates in the range of 75-95% could be achieved by either surgery alone or radiation therapy (RT) alone (2-4). RT generally has the advantages of wider applicability and higher likelihood of preserving serviceable voice quality than surgery (5). Several factors affecting the clinical outcomes have been reported in RT, including the dose per fraction and the dose schedules (6). Authors retrospectively analyzed the clinical outcomes and the influences of possible prognostic factors in early glottic cancer treated by two different radiation dose schedules, and would report our experience.
From February of 1995 till June of 2008, the number of patients with T1-2N0M0 glottic caner, who were given curative RT, was 157. The staging work-up included thorough history and physical examination, chest X-ray, neck CT scans, PET or PET-CT scans, and routine blood tests. All the patients had squamous cell carcinoma and underwent laryngomicroscopy for histologic confirmation.
After taking CT simulation at the treatment posture, the radiation dose plan was made on each individual patient. The radiation target volume routinely included the upper border of the thyroid cartilage at the top and the lower border of the cricoid cartilage at the bottom, and minimum of 1-1.5 cm margins were put around all the clinically known disease extent of the individual patient. Anterior paired wedge beam arrangement with 4- or 6-MV photons from linear accelerators were used in all the patients. The radiation portal sizes varied from 5├Ś5 cm2 to 7├Ś8 cm2 depending on the patients' contour. Two different radiation dose schedules were used along the study duration: 70 Gy in 35 fractions over 7 weeks by daily 2.0 Gy to 64 patients (40.8%) treated from February of 1995 till May of 2001 (group A); and 67.5 Gy in 30 fractions over 6 weeks by daily 2.25 Gy to 93 patients (59.2%) treated from June of 2001 till June of 2008 (group B). The biologically effective doses (BED) of acute and late responding tissues were similar between the groups: 84.0 Gy10 and 116.7 Gy3 in the group A; and 82.7 Gy10 and 118.1 Gy3 in the group B.
Follow-up evaluation with laryngoscopy and physical examination were done regularly on an out-patient basis, and the response evaluation was done in 1 month of completion of RT. The follow-up intervals were 2-3 months'= during the first year, 3-6 months' during the second to third years, and 6-12 months thereafter. Chest X-rays were taken at least once a year, and CT scan, PET-CT or other laboratory tests were done whenever clinically indicated. The events of side effects, recurrence or metastasis, death, and occurrence of the second malignancy were recorded during the follow-up.
The dates of the RT start were the bases of all the observation durations. The survival rates were calculated by Kaplan-Meier method, and their comparisons were done by log-rank test. The comparisons among the groups were done by Student t-test, and Cox regression test was used for multivariate analysis.
The patients' age ranged from 35 years to 83 years with the median age of 62 years, with the male preponderance (151, 96.2%). The laryngomicroscopy was done only for diagnostic evaluation and biopsy in 116 patients (73.8%), and for potentially curative resection in 41 (26.2%). Clinical T-stages based on laryngomicroscopic evaluation were T1a in 89 patients (56.7%), T1b in 36 (22.9%), and T2 in 32 (20.4%), respectively. The median overall treatment durations were 50 days (range, 47 to 59 days) and 44 days (range, 40 to 67 days) in the groups A and B. There was no significant difference in the distribution of the characteristics between the treatment groups, except sex (Table 1).
Vast majority of the patients experienced radiation mucositis and dermatitis presenting from 2 weeks of RT start, and complained of throat pain on swallowing and voice change. These acute side effects were, however, grade 2 or lower, and controlled with symptomatic management including analgesics medication. There was no significant difference in grade 2 mucositis incidence between the groups: two in the group A (3.1%); and six in the group B (6.5%). No delayed complication of grade 2 or higher was observed during the follow-up.
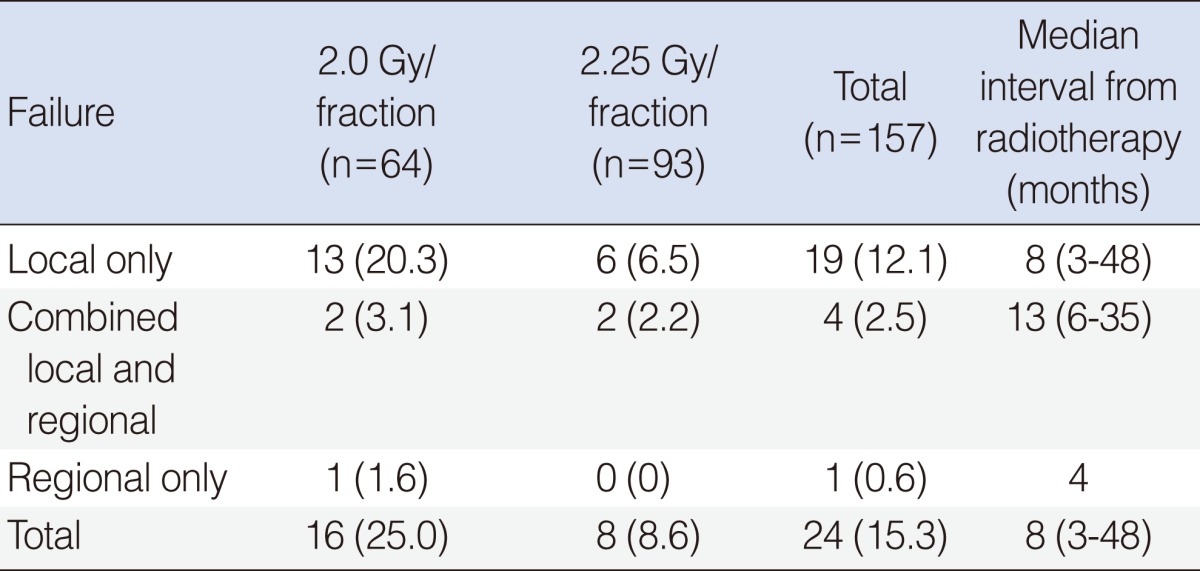
The median follow-up durations were 85 and 45 months in the groups A and B, respectively. Local control, evaluated in 1 month of completion of RT, was achieved in all the patients. Recurrence, however, was observed in 24 patients (15.3%) during the follow-up: local relapse only in 19 patients (12.1%) at the median follow-up of 8 months (range, 3 to 48 months); combined local and regional recurrence in four (2.5%) at the median follow-up of 13 months (range, 6 to 35 months); and regional recurrence only in one (0.6%) at 4 months (Table 2). There was no occurrence of distant metastasis during the follow-up. Twenty patients underwent salvage surgery, three received re-irradiation, and one abandoned further salvage therapy. Total laryngectomy was performed in seven patients.
The disease-free, overall, and disease-specific survival rates at 5 years in all the patients were 84.7%, 92.6%, and 94.8% (Fig. 1). The tumor extent, vocal cord motility, T-stage, and the radiation dose schedule were the significantly important factors affecting the disease-free survival on univariate analyses, while the tumor extent and the radiation dose schedule remained as significant factors on multivariate analyses (Table 3). The disease-free survival rates at 5 years were 78.3% and 90.8% in the groups A and B. There was a significant difference in the disease-free survival rates in T1 stage (83.4% vs. 94.6%, P=0.025), but not in T2 stage (62.7% vs. 60.6%, P=0.965) based on the treatment groups (Fig. 2). The disease-free survival rate at 5 years in those who received salvage therapy for recurrence was 75.0%.
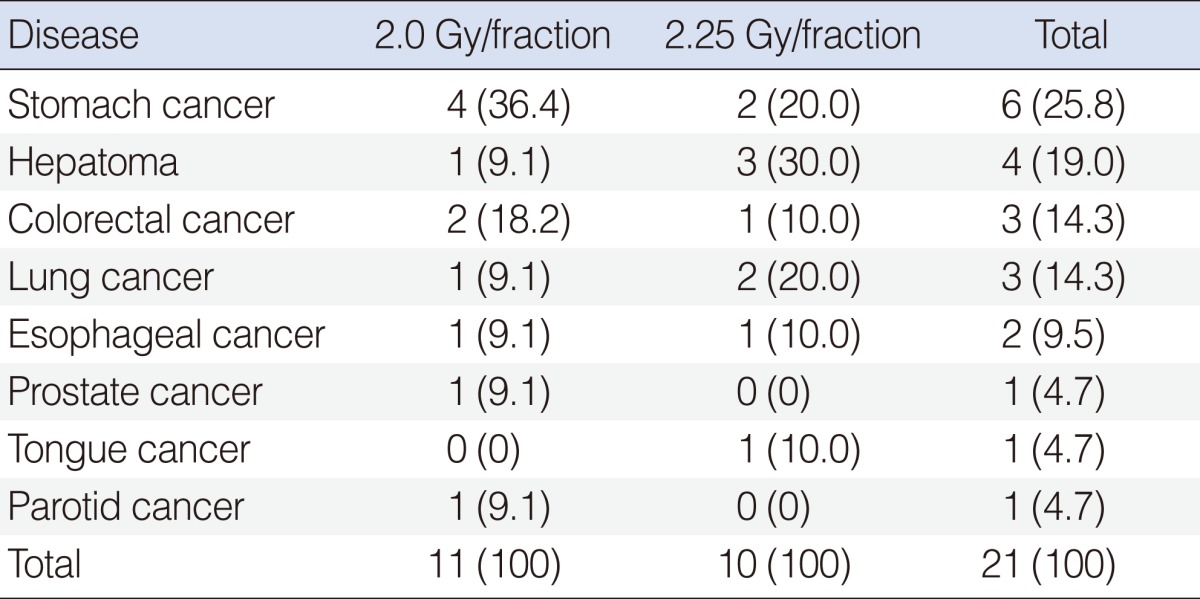
The second malignancy was diagnosed in 21 patients during the follow-up: stomach cancer in six (25.8%); hepatoma in four (19.0 %); colorectal cancer in three (14.3%); lung cancer in three (14.3%); esophageal cancer in two (9.5%); prostate cancer in one (4.7%); tongue cancer in one (4.7%); and parotid cancer in one (4.7%) (Table 4). There was no difference in the incidence of second malignancy based on the radiation dose schedules.
In treating early glottic cancer, therapeutic options with the capability of voice preservation include open partial laryngectomy, transoral laser excision, and RT. Favorable outcomes could be achieved by any of these modalities, and the local control rates at 5 years ranged from 85% to 95% in T1, and from 60% to 80% in T2 stages (2-4, 7-9). The choice of treatment modality usually depends on T-stage, anterior commissure involvement, preference of patient and physician, general status of patient (5, 10). Among these therapies, RT and transoral laser excision are equally recommended for early glottic cancer in general. However, RT may be preferred to surgery if the tumor size becomes bigger, considering the post-therapy voice quality, while open partial laryngectomy is usually considered for treating local recurrence following other modalities (5).
Usual recommendation in RT for early glottic cancer was to give higher radiation dose to T2 stage than to T1. T2 stage differs from T1 in two aspects: the extent of tumor outside the true vocal cord; and/or the impaired vocal cord motility. These rather arbitrary differentiation points, however, are subject to inter-observer variation and not always reliable even after laryngomicroscopic evaluation under general anesthesia. The current study employed the same dose schedules to both T1 and T2 stages assuming that there might have been, more or less, subjective variations in the T-stage assignment, and better local control rates at 5 years were achieved in T1 stage than in T2 (90.2% vs. 61.4%, P<0.001). Our local control rates, especially in T1 stage, could be favorably comparable to the other reports that usually applied differential total doses based on the clinical T-stages. This implies that there might have been a dose-response relationship within T1 stage disease (11), and that the stage assignment, if based mainly on the visual evaluation of the tumor extent and the cord motility, has the tendency of understaging of T2 disease to T1.
Several patient-related prognostic factors with respect to the local control by RT were reported, and included histologic differentiation, pre-therapy hemoglobin level, p53 expression, T-stage, anterior commissure involvement, vocal cord motility, and tumor extent. The prognostic significance of the tumor extent, vocal cord motility, T-stage, and the radiation dose schedule on the disease-free survival were affirmed by the current study. Some reported that the anterior commissure involvement was related with the increased risks of local failure and necrosis following either RT or surgery, however, there were controversial opinions (5, 6, 12-14). No significance was seen based on the anterior commissure involvement in the current study.
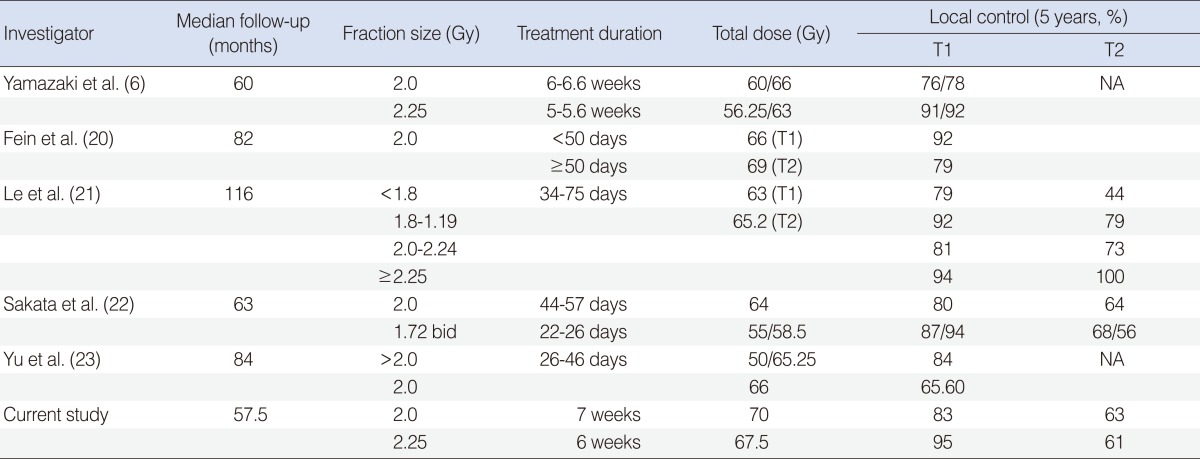
Among the several RT-related prognostic factors, the duration of RT and the fractional radiation dose are very important. It was reported that the tumor doubling time in head and neck cancers could be as short as 3-5 days during the anti-cancer chemotherapy or RT courses, which could lead to unfavorable local control (15, 16). To overcome the accelerated repopulation, shortening of the RT duration is desirable. Early glottic cancer, as it needs relatively small and limited radiation volume, could serve an ideal model to investigate the influence of the RT duration over the local control rate. The RT duration and the fraction size are very closely related, and the risk of delayed morbidity should be carefully considered when increasing the fractional radiation dose when shortening the RT duration. Several studies already suggested that the fractional dose higher than 3.0 Gy could lead to unacceptably high level of severe complications (17-19). Since 1990, there have been several reports on the fraction size and the local control rate (Table 5) (6, 20-23). Le et al. (21) applied 4 different fraction sizes (<180 cGy, 180-199 cGy, 200-224 cGy, and Ōēź225 cGy), and found there was no difference in local control rates in T1 stage, while significantly better local control achieved in T2 stage Ōēź by 225 cGy (100%) when compared with <180 cGy (44%). Yu et al. (23) reported the similar effect in T1 stage (84% by >200 cGy/fraction and 65.5% by 200 cGy/fraction). Yamazaki et al. (6) prospectively compared the impact of the fraction sizes, and reported that the local control rates were 77% by 200 cGy and 92% by 225 cGy with no significant difference in the incidence of side effects. Rudoltz et al. (24) reported decreasing local control rates with increasing RT durations: 100% if RT was completed within 42 days; 91% for 43-46 days; 74% for 47-50 days; 65% for 51-54 days; and 50% for 55-66 days (P=0.0001). Fein et al. (20) also reported that the RT duration shorter than 50 days was advantageous with respect to the local control rates at 2 years (92% vs. 82%, P=0.07). The local control rates in the current study were favorably comparable to the others, while there was no increase of the complication rates according to the fractional doses with no grade 3 or higher side effects.
The treatment failures after RT for early glottic cancer were known to occur mostly in the local area, while the regional failure rates were 5-20% (25, 26), and surgery should be considered for salvaging the loco-regional failures following RT. Nibu et al. (27) applied partial laryngectomy as salvage modality, and reported the 5-year survival and larynx-preservation rates of 86%. Le et al. (21), following transoral laser surgery or open partial laryngectomy, reported the local control rates of 83% in T1 stage, and 74% in T2 stage, and the ultimate local control rates at 10 years of 96% in T1, and 91% in T2 stages. In the current study, there were total of 24 patients with loco-regional recurrences among 157 patients (crude rate, 15.3%), and 3/4 of them were salvaged following salvage therapy mainly by surgery. As seven patients underwent total laryngectomy, the crude larynx-preservation rate among the entire patients was 95.5%.
Surveillance of the second malignancy is very important as the patients should have already been exposed to the carcinogens of the upper aerodigestive tracts, and they have high expectancy of long-term survival following RT. The reported incidence of the second malignancy is in the range of 20% to 25%, which usually becomes greater with longer follow-up duration (28, 29). Franchin et al. (30) reported that the incidence of the second malignancy among the patients with early glottic cancer was 22.2%, commonly involving the respiratory tract, the head/neck, and the uro-genital tract. The second cancer incidence reported by Yamazaki et al. (6) was 25%, where the stomach, the head/neck, the esophagus, and the lung were commonly involved. The authors observed 21 patients who developed the second malignancy (13.3%) at median 37 months' follow-up, and this incidence might increase with longer-term follow-up. The organs of the second malignancy in the current study were the stomach, the liver, the colorectum, and the lung, which was more or less similar to Japanese reports.
RT traditionally has served as a standard therapy for early glottic cancer with the high local control rates, a favorable voice quality, and the low complication rates. In the current study, the vocal cord mobility, the tumor extent and the fraction size were the significant factors for the disease-free survival. With the fractional dose of 2.25 Gy, instead of 2.0 Gy per fraction, the authors could deliver the equivalent biologic total dose during shorter duration, and achieve improved disease-free survival in the T1 stage at no increased toxicity.
References1. DeVita VT, Lawrence TS, Rosenberg SA. DeVita, Hellman, and Rosenberg's cancer: principles & practice of oncology. 2008. 8th ed. Philadelphia: Lippincott Williams & Wilkins.
2. Akimoto T, Nonaka T, Kitamoto Y, Ishikawa H, Ninomiya H, Chikamatsu K, et al. Radiation therapy for T2N0 laryngeal cancer: a retrospective analysis for the impact of concurrent chemotherapy on local control. Int J Radiat Oncol Biol Phys. 2006 3;64(4):995-1001. PMID: 16406396.
3. Kim WT, Nam JH, Kyuon BH, Wang SG, Kim DW. Radiotherapy for early glottic carinoma. J Korean Soc Ther Radiol Oncol. 2002 12;20(4):295-302.
4. Lee HS, Moon SR, Ahn KJ, Chung EJ, Suh CO, Kim GE, et al. The heterogeneity of T2NO glottic carcinoma treated by irradiation. J Korean Soc Ther Radiol. 1990 12;8(2):199-206.
5. Mendenhall WM, Werning JW, Hinerman RW, Amdur RJ, Villaret DB. Management of T1-T2 glottic carcinomas. Cancer. 2004 5;100(9):1786-1792. PMID: 15112257.
6. Yamazaki H, Nishiyama K, Tanaka E, Koizumi M, Chatani M. Radiotherapy for early glottic carcinoma (T1N0M0): results of prospective randomized study of radiation fraction size and overall treatment time. Int J Radiat Oncol Biol Phys. 2006 1;64(1):77-82. PMID: 16169681.
7. Garden AS, Forster K, Wong PF, Morrison WH, Schechter NR, Ang KK. Results of radiotherapy for T2N0 glottic carcinoma: does the "2" stand for twice-daily treatment? Int J Radiat Oncol Biol Phys. 2003 2;55(2):322-328. PMID: 12527044.
8. McCoul ED, Har-El G. Meta-analysis of impaired vocal cord mobility as a prognostic factor in T2 glottic carcinoma. Arch Otolaryngol Head Neck Surg. 2009 5;135(5):479-486. PMID: 19451470.
9. Park JH, Paeng JP, Na HS, Lim KJ, Kwon SY, Jung KY, et al. Treatment results of laser cordectomy and radiation therapy for early glottic cancer. Korean J Otolaryngol-Head Neck Surg. 002 2;45(2):159-163.
10. Dey P, Arnold D, Wight R, MacKenzie K, Kelly C, Wilson J. Radiotherapy versus open surgery versus endolaryngeal surgery (with or without laser) for early laryngeal squamous cell cancer. Cochrane Database Syst Rev. 2002;(2):CD002027PMID: 12076435.
11. Agrawal N, Ha PK. Management of early-stage laryngeal cancer. Otolaryngol Clin North Am. 2008 8;41(4):757-769. PMID: 18570957.
12. Marshak G, Brenner B, Shvero J, Shapira J, Ophir D, Hochman I, et al. Prognostic factors for local control of early glottic cancer: the Rabin Medical Center retrospective study on 207 patients. Int J Radiat Oncol Biol Phys. 1999 3;43(5):1009-1013. PMID: 10192348.
13. Nozaki M, Furuta M, Murakami Y, Izawa Y, Iwasaki N, Takahashi H, et al. Radiation therapy for T1 glottic cancer: involvement of the anterior commissure. Anticancer Res. 2000;Mar-Apr;20(2B):1121-1124. PMID: 10810406.
14. Wolfensberger M, Dort JC. Endoscopic laser surgery for early glottic carcinoma: a clinical and experimental study. Laryngoscope. 1990 10;100(10 Pt 1):1100-1105. PMID: 2215043.
15. Fowler JF. Fractionated radiation therapy after Strandqvist. Acta Radiol Oncol. 1984;23(4):209-216. PMID: 6093436.
16. Horiot JC, Bontemps P, van den Bogaert W, Le Fur R, van den Weijngaert D, Bolla M, et al. Accelerated fractionation (AF) compared to conventional fractionation (CF) improves loco-regional control in the radiotherapy of advanced head and neck cancers: results of the EORTC 22851 randomized trial. Radiother Oncol. 1997 8;44(2):111-121. PMID: 9288839.
17. Harwood AR, Tierie A. Radiotherapy of early glottic cancer-II. Int J Radiat Oncol Biol Phys. 1979 4;5(4):477-482. PMID: 457492.
18. Randall ME, Springer DJ, Raben M. T1-T2 carcinoma of the glottis: relative hypofractionation. Radiology. 1991 5;179(2):569-571. PMID: 2014313.
19. van der Voet JC, Keus RB, Hart AA, Hilgers FJ, Bartelink H. The impact of treatment time and smoking on local control and complications in T1 glottic cancer. Int J Radiat Oncol Biol Phys. 1998 9;42(2):247-255. PMID: 9788401.
20. Fein DA, Lee WR, Hanlon AL, Ridge JA, Curran WJ, Coia LR. Do overall treatment time, field size, and treatment energy influence local control of T1-T2 squamous cell carcinomas of the glottic larynx? Int J Radiat Oncol Biol Phys. 1996 3;34(4):823-831. PMID: 8598359.
21. Le QT, Fu KK, Kroll S, Ryu JK, Quivey JM, Meyler TS, et al. Influence of fraction size, total dose, and overall time on local control of T1-T2 glottic carcinoma. Int J Radiat Oncol Biol Phys. 1997 8;39(1):115-126. PMID: 9300746.
22. Sakata K, Someya M, Hori M, Nakata K, Takagi M, Hareyama M. Hyperfractionated accelerated radiotherapy for T1,2 glottic carcinoma: consideration of time-dose factors. Strahlenther Onkol. 2008 7;184(7):364-369. PMID: 19016035.
23. Yu E, Shenouda G, Beaudet MP, Black MJ. Impact of radiation therapy fraction size on local control of early glottic carcinoma. Int J Radiat Oncol Biol Phys. 1997 2;37(3):587-591. PMID: 9112457.
24. Rudoltz MS, Benammar A, Mohiuddin M. Prognostic factors for local control and survival in T1 squamous cell carcinoma of the glottis. Int J Radiat Oncol Biol Phys. 1993 8;26(5):767-772. PMID: 8344844.
25. Kim YH, Chai GY. Radiotherapy of early stage glottic cancer. J Korean Soc Ther Radiol. 1997 12;15(4):315-320.
26. Mendenhall WM, Amdur RJ, Morris CG, Hinerman RW. T1-T2N0 squamous cell carcinoma of the glottic larynx treated with radiation therapy. J Clin Oncol. 2001 10;19(20):4029-4036. PMID: 11600604.
27. Nibu K, Kamata S, Kawabata K, Nakamizo M, Nigauri T, Hoki K. Partial laryngectomy in the treatment of radiation-failure of early glottic carcinoma. Head Neck. 1997 3;19(2):116-120. PMID: 9059868.
28. Fujita M, Rudoltz MS, Canady DJ, Patel P, Machtay M, Pittard MQ, et al. Second malignant neoplasia in patients with T1 glottic cancer treated with radiation. Laryngoscope. 1998 12;108(12):1853-1855. PMID: 9851503.
29. Narayana A, Vaughan AT, Fisher SG, Reddy SP. Second primary tumors in laryngeal cancer: results of long-term follow-up. Int J Radiat Oncol Biol Phys. 1998 10;42(3):557-562. PMID: 9806515.
30. Franchin G, Minatel E, Gobitti C, Talamini R, Vaccher E, Sartor G, et al. Radiotherapy for patients with early-stage glottic carcinoma: univariate and multivariate analyses in a group of consecutive, unselected patients. Cancer. 2003 8;98(4):765-772. PMID: 12910521.
Fig.┬Ā1Disease-free survival (A), overall survival (B), and disease-specific survival (C) of the patients with early glottic cancer treated by definitive radiation therapy. 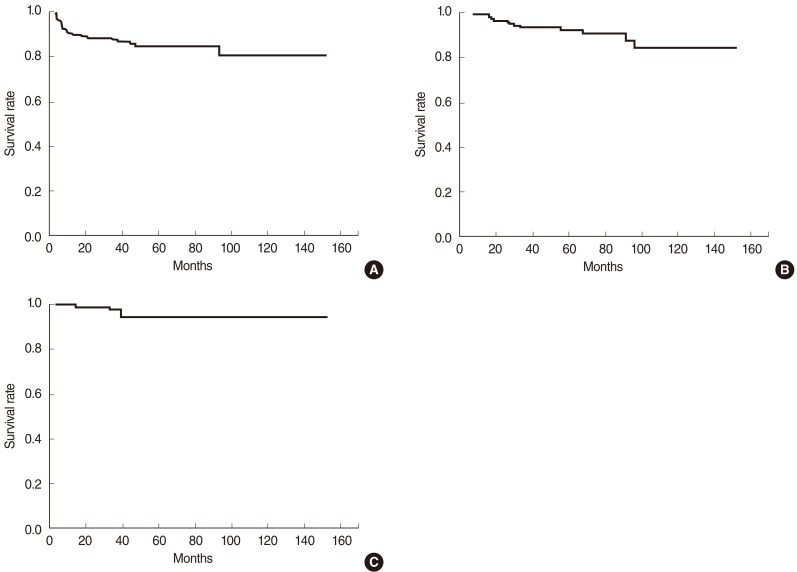
Fig.┬Ā2Impact of fraction size by T-stage: disease-free survival rates at 5 years were 83.4% and 94.6% in the groups A and B of T1 patients (P=0.025) (A), and 62.7% and 60.6% in the groups A and B of T2 patients (P=0.965), respectively (B). 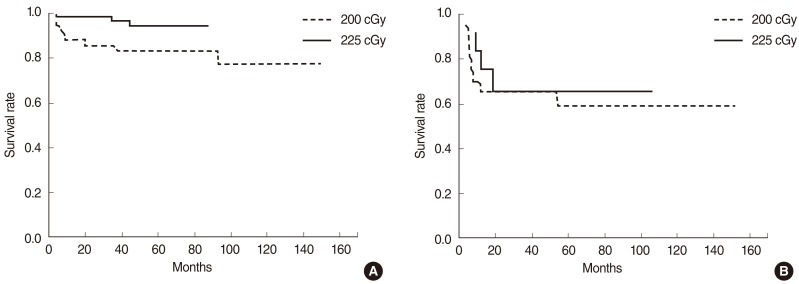
|
|
||||||||||||||||||||||||||||||||||||||