|
 |
- Search
AbstractObjectivesAllergic rhinitis (AR) is a chronic upper respiratory tract disease that inflames the mucous membranes of the nose and occurs when circulating inflammatory cells including eosinophils and basophils migrate to and accumulate in the inflammation area by passing through the interstitium and capillary walls. To pass through these barriers, the inflammatory cells degrade extracellular matrix proteins. Matrix metalloproteinases (MMPs) released by inflammatory cells mediate the degradation of these proteins. MMPs have synthetic inhibitors and doxycycline, a tetracycline antibiotic, inhibits MMPs. This study investigated the efficiency of intranasal doxycycline in decreasing the symptoms and inflammatory cell infiltration in an animal model of AR.
MethodsAR was created in female Wistar rats by repeated intranasal challenge with ovalbumin by intraperitoneal injection. For 15 days, topical intranasal doxycycline was administered one hour before ovalbumin administration. Following intranasal administration, nasal symptoms were scored and the nasal mucosae of all rats were evaluated histopathologically. To investigate tissue changes, hematoxyline-eosin and Alcian blue/periodic acid Schiff stains were used. As well, cilia loss, goblet cell changes, vascular congestion, vascular proliferation, inflammatory cell infiltration, eosinophil infiltration and the degree of hypertrophy in chondrocytes were evaluated with light microscopy.
Allergic rhinitis (AR) is a chronic upper respiratory tract disease that involves inflammation of the mucous membranes of the nose. AR occurs when circulating inflammatory cells including eosinophils and basophils migrate to and accumulate in the inflammation area by passing through the interstitium and capillary walls [1,2]. To pass through these barriers, inflammatory cells degrade extracellular matrix (ECM) proteins. The degradation of these proteins is mediated by matrix metalloproteinases (MMPs) released by inflammatory cells [3,4]. MMPs are a multigenic family of endopeptidases whose synthesis, secretion and activation are regulated by transcriptional regulation, latent enzyme activation and specific tissue inhibitors of metalloproteinases (TIMPs) [3]. Besides TIMPs, some synthetic inhibitors of MMPs function by binding to Zn++ atoms. The tetracycline antibiotic doxycycline (Dox) inhibits MMP activity at sub-antimicrobial doses without any side effects [5].
This study investigated the efficiency of intranasal Dox in decreasing the symptoms of AR and mucosal histological changes in an animal model.
This study was conducted on 21 healthy female Wistar rats weighing between 250 g and 300 g, which had been bred in the Center of Laboratory Animal Breeding and Experimental Studies at Dokuz Eylul University. The rats were housed in 30├Ś18├Ś24 cm cages (four rats per cage) in a 12 hours light/12 hours dark schedule at 24Ōäā┬▒2Ōäā and fed standard pellet diet. The animals had free access to food and water. The study was approved by the Committee on Ethics in Animal Experimentation of Dokuz Eylul University (approval number: 30/2009, approval date: June 5, 2009).
After acclimatization to laboratory conditions, the first phase of the study involved the immunization of the rats. Fourteen rats were sensitized with ovalbumin (OVA; 0.3 mg intraperitoneally; Grade V, Sigma-Aldrich, St. Louis, MO, USA) as antigen, which was administered together with aluminum hydroxide (30 mg) in saline (1 mL intraperitoneally) every 2 days for 14 days, between 11 and 12 AM. In the second phase, all 14 sensitized animals were used to develop a model of AR by repeated intranasal instillation of 10 ┬ĄL of 20 mg/mL OVA with a micropipette daily for 15 days between 11 and 12 AM [6]. The animals were separated into two groups. Group A included 7 sensitized rats (AR group). The 7 rats in group B additionally received 10 ┬ĄL of 20 mg/mL Dox (D-9891; Sigma-Aldrich) dissolved in 0.9% physiological saline, which was instilled into both nostrils using a pipette one hour before the administration of OVA for 15 days (Dox group). Additionally, a control group (group C) was established; 7 rats were repeatedly sensitized and challenged with saline with the same dose and on a similar time schedule as the other animals in the other groups.
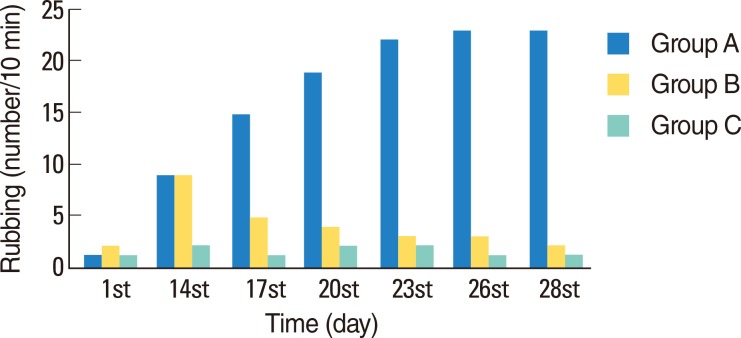
The immediate and late reactions of all animals after nasal challenge were examined on day 1, 14, 17, 20, 23, 26, and 28 after the administration OVA. Observations were performed by the same person for 10 minutes, following an adaptation period of 10 minutes. AR was evaluated regarding the severity of typical clinical symptoms that included nasal irritation, sneezing and nasal secretion. Nasal symptom scores were graded on a four-point scale, each grade was assigned a numerical score (0-3) and the scores were graded as summarized in Table 1. On day 14, which was the first day of sensitization, nasal scratching, sneezing and nasal flow were scored. The AR model was considered successful if the total score exceeded 5 [6].
All animals were sacrificed by 80 mg/kg intraperitoneal pentobarbital injection and 100 mL 0.9% physiological saline perfusion followed by 400 mL formaldehyde introduced through the left ventricle 24 hours after the last intranasal challenge. The head of each rat was removed and fixed in 10% neutral buffered formalin for 3 days, then decalcified in 5% trichloroacetic acid for 7 days. The nasal cavity was transversely sectioned at the level of incisive papilla of the hard palate and the tissue block was embedded in paraffin. Some of the sections were stained with hematoxylin and eosin (H&E) to examine tissue histology and the remaining sections were stained with Alcian blue and periodic acid Schiff (AB/PAS) to highlight goblet cells. Digital images were obtained from bothstained sections using a model DP71 camera (Olympus Optical, Tokyo, Japan) connected to a model BX51 light microscope (Olympus Optical) at an original magnification of ├Ś40. Images were processed with the 3D Reconstruct Program 1.1.0.1 (Medical College of Georgia, Georgia Health Sciences University, Augusta, GA, USA). Cilia loss, goblet cell increase, vascular congestion, vascular proliferation, inflammatory cell infiltration, eosinophil infiltration, and the degree of hypertrophy in chondrocytes were evaluated [7,8,9] in each section. The change in each parameter was scored as 0, no change; 1, mild change; 2, moderate change; or 3, severe change. All histomorphological analyses were performed by two histologists with no prior knowledge of the treatment groups.
The mean of each parameter was statistically analyzed using SPSS ver. 15.0 (SPSS, Chicago, IL, USA). The analyses were performed by assigning the results into groups as none to mild (scores below 1.5) and moderate to severe (scores above 1.5). Fisher exact test was used to test for the differences between the groups. The measurement of sneezes and nasal scratches was evaluated using Mann-Whitney U-test for paired groups. Groups were compared within themselves using Wilcoxon signed-rank test, P<0.05 was considered significant in all analyses.
Following injection and initial intranasal challenge with OVA, typical AR symptoms including frequent sneezing, nasal scratching and watery rhinorrhea were observed in each animal, which then gradually disappeared over approximately 1.5 hours. Since the total score in all animals was >5 at the first subjective evaluation of AR carried out on day 14, the AR model was considered successful. Thus, all animals in groups A, B, and C were included in the study. Subsequent intranasal challenges with OVA triggered more severe symptoms that persisted longer. However, symptoms were reduced and disappeared on day 2 in animals treated with Dox. Furthermore, subsequent intranasal challenges with OVA did not trigger AR symptoms.
In group A, the number of sneezes was considerably higher on days 14, 17, 20, 23, 26, and 28 than on day 1 (P=0.017, P=0.017, P=0.017, P=0.016, P=0.016, and P=0.017, respectively). In group B (Dox-treated), a decrease in the number of sneezes was observed, beginning from day 3 of treatment (Fig. 1). Comparison of groups A and B revealed no significant differences between the number of sneezes among the two groups on day 1 and 14 (P=0.606 and P=0.790, respectively). However, compared to group A, a decline was observed in the mean number of sneezes in group B on day 17, 20, 23, 26, and 28. The differences between the two groups were significant (P=0.001, P=0.001, P=0.001, P=0.001, and P=0.001, respectively). Throughout the study, a significant difference in number of sneezes was not observed in group C.
Statistically significant differences were not observed between the mean number of nose scratches among the groups A and B on day 1 and 14 (P=0.728 and P=0.181, respectively). However, a significant decrease in the number of nasal scratches was observed in the Dox-treated rats in group B on day 17, 20, 23, 26 and 28 (P=0.002, P=0.001, P=0.001, P=0.001, and P=0.001, respectively). In group C, no significant differences were observed in the number of nose scratches on the aforementioned days (Fig. 2).
Semiquantitative histological evaluation of the nasal cavity, nasal septum and lateral nasal walls revealed that there were normal histological structures in the control group with regular ciliae and goblet cells in the pseudostratified ciliated cylindrical respiratory epithelium cells. Eosinophil and inflammatory cell infiltration were not observed in the lamina propria, connective tissue structures were normal, and vascular congestion and proliferation was not present and mucosal glands and chondrocytes were normal (Fig. 3, Table 2).
Significant changes were observed for all histological parameters in group A. H&E staining demonstrated cilia loss in the respiratory epithelium cells; eosinophil and inflammatory cell infiltration and vascular congestion and proliferation in the connective tissue veins were significantly higher in this group of rats than in the control group (P=0.001, P=0.001, P=0.001, P= 0.001, P=0.001, respectively). Examination of the cartilage tissues revealed significant hypertrophy in the chondrocytes (P=0.001) (Fig. 4). AB/PAS staining revealed a significant increase in goblet cells (P=0.001). The results of the HE staining in group B demonstratedminimal cilia loss in the respiratory epithelium cells, also eosinophil and inflammatory cell infiltration and vascular congestion and proliferation in the connective tissue veins were significantly reduced, compared to group A (P=0.005, P=0.001, P=0.001, P=0.001, P=0.001, P=0.001, respectively). Although AB/PAS staining demonstrated a decline in the goblet cell increase, a statistically significant difference was not present between groups A and B (P=1.000). There were no significant differences between group B and the control groupwith respect to the results of the H&E staining (all P=1.000). The goblet cell count revealed by AB/PAS staining in group B was found to be close to that in the control group, although it was significantly higher (P=0.001).
The search for the treatment of AR has led to various attempts to establish animal models and investigations of the effects of certain drugs. In humans, the main symptoms of AR are sneezing, itchy nose and rhinorrhea; therefore, there is a need for animal models that exhibit similar allergic symptoms. This study clearly revealed that repeated topical intranasal OVA application causes typical AR symptoms such as sneezing and nose scratching in rats and provides a good model for experimental AR [6,10].
Immunohistological studies aimed at investigating the nasal tissues of AR patients have indicated intense accumulation of eosinophils and basophil/mast cells in the lamina propria and epithelium [11]. The recruitment and migration of these inflammatory cells to sites of inflammatory reactions involves traversing the capillary walls and the interstitium [1,2,3]. To traverse these barriers, inflammatory cells adhere and degrade ECM proteins [4,5,6]. The degradation of ECM proteins by inflammatory cells is accomplished in part by the secretion of MMPs, such as MMP-2 and MMP-9, which specifically shred denatured collagen, native type IV and V collagens and elastin [12,13]. In murine models, inflammatory cells, MMP-9, intercellular adhesion molecule (ICAM)-1, vascular cell adhesion molecule (VCAM)-1 protein and mRNA levels are increased after challenge with toluene diisocyanate. The administration of a MMP inhibitor reportedly reduced the amount of inflammatory cells and the levels of ICAM-1 and VCAM-1 mRNA and protein, supporting the notion that MMP inhibitors inhibit inflammatory cell migration by causing a decrease in adhesion molecules [3,4].
Introduction of platelet activating factor and interleukin can prompt eosinophil transmigration from the artificial basal membrane and the introduction of Batimastat, a synthetic MMP inhibitor, can inhibit this transmigration in vitro [14]. The present in vivo observations of significant decreases of eosinophils and infiltration of other inflammatory cells in the nasal mucosa of Dox-treated rats, support the in vitro results and strengthen the validity of the animal model.
Structural abnormalities seen in AR, which are collectively termed tissue remodeling, are caused by MMPs secreted from epithelial cells and fibroblasts in addition to infiltrating inflammatory cells [15]. MMPs are also reportedly responsible for microvascular permeability leading to edema, cell migration and ECM remodelling at the site of inflammation [2]. Therefore it is reasonable to speculate that manipulation of MMP production from inflammatory cells, epithelial cells and fibroblasts by anti-allergic agents may be an important strategy for treating symptoms of allergic diseases including AR. In this study, cilia loss in the respiratory epithelium cells, marked vascular congestion and proliferation in the veins located in the connective tissue, significant hypertrophy in the chondrocytes and a significant increase in goblet cells were demonstrated in AR rats. However, in the Dox-treated group, ciliary loss, vascular congestion and proliferation, chondrocyte hypertrophy and increase in goblet cells were reduced significantly.
Fluticasone propionate, a H1-receptor-antagonist, can reduce the release of MMP-2 and MMP-9, and the expression of MMP-mRNA in nasal fibroblasts, demonstrating that the action of corticosteroids in the treatment of AR is mediated by MMPs [16]. To investigate the effects of fexofenadine hydrochloride on MMPs, nasal fibroblasts were stimulated in vitro by tumor necrosis factor-alpha. The compound reduced the release of MMP-2 and MMP-9 and the expression of MMP-mRNA in a similar fashion [3]. Dox inhibits MMP activity at sub-antimicrobial doses [5,17]. This study is the first to administer topical intranasal Dox for a therapeutic purpose in an AR model. Treatment with 20 mg/mL Dox caused a significant decrease in AR symptoms including nasal scratching and sneezing, and that the repeated administration of OVA does not cause an increase in these symptoms. The histological examinations carried out after Dox treatment revealed a significant decrease in the typical inflammatory changes seen in AR. Our results support the view that MMPs may have crucial functions in AR and topical intranasal Dox, which decreases inflammatory cell infiltration, may be a treatment option in AR.
The main limitations of this preliminary study that we did not study OVA-specific IgE to confirm the establishment of AR model and did not evaluate cytokines related with AR. Additionally, evaluating the MMP expression using immunohistochemical staining of MMPs would be helpful to explain the role of Dox. A multicentric, double-blinded and randomized controlled clinical trials investigating the evidence acquired from our experimental animal model may help provide a new option in the treatment of AR.
References1. Golub LM, Lee HM, Ryan ME, Giannobile WV, Payne J, Sorsa T. Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Adv Dent Res. 1998 11;12(2):12-26. PMID: 9972117.
2. Asano K, Kanai KI, Suzaki H. Suppressive activity of fexofenadine hydrochloride on metalloproteinase production from nasal fibroblasts in vitro. Clin Exp Allergy. 2004 12;34(12):1890-1898. PMID: 15663564.
3. De S, Fenton JE, Jones AS. Matrix metalloproteinases and their inhibitors innon-neoplastic otorhinolaryngological disease. J Laryngol Otol. 2005 6;119(6):436-442. PMID: 15992468.
4. Lee KS, Jin SM, Kim HJ, Lee YC. Matrix metalloproteinase inhibitor regulatesinflammatory cell migration by reducing ICAM-1 and VCAM-1 expression in a murine model of toluene diisocyanate-induced asthma. J Allergy Clin Immunol. 2003 6;111(6):1278-1284. PMID: 12789230.
5. Baltac─▒oglu E, Akal─▒n A. Tetracyclines and their non-antimicrobial properties: a new approach to their use in periodontal treatment. Hacettepe Di┼¤hekimli─¤i Fak├╝ltesi Dergisi. 2006;30(1):97-107.
6. Wen WD, Yuan F, Wang JL, Hou YP. Botulinum toxin therapy in the ovalbumin-sensitized rat. Neuroimmunomodulation. 2007;14(2):78-83. PMID: 17713354.
7. Salib RJ, Howarth PH. Remodelling of the upper airways in allergic rhinitis: is it a feature of the disease? Clin Exp Allergy. 2003 12;33(12):1629-1633. PMID: 14656347.
8. Bousquet J, Jacot W, Vignola AM, Bachert C, Van Cauwenberge P. Allergic rhinitis: a disease remodeling the upper airways? J Allergy Clin Immunol. 2004 1;113(1):43-49. PMID: 14713906.
9. Ercan I, Cakir BO, Basak T, Ozbal EA, Sahin A, Balci G, et al. Effects oftopical application of methotrexate on nasal mucosa in rats: a preclinicalassessment study. Otolaryngol Head Neck Surg. 2006 5;134(5):751-755. PMID: 16647529.
10. Shimizu S, Hattori R, Majima Y, Shimizu T. Th2 cytokine inhibitor suplatasttosilate inhibits antigen-induced mucus hypersecretion in the nasal epithelium of sensitized rats. Ann Otol Rhinol Laryngol. 2009 1;118(1):67-72. PMID: 19244966.
11. Bentley AM, Jacobson MR, Cumberworth V, Barkans JR, Moqbel R, Schwartz LB, et al. Immunohistology of the nasal mucosa in seasonal-allergic rhinitis: increases in activated eosinophils and epithelial mast cells. J Allergy Clin Immunol. 1992 4;89(4):877-883. PMID: 1532808.
12. Herouy Y, Mellios P, Bandemir E, Dichmann S, Nockowski P, Schopf E, et al. Inflammation in stasis dermatitis upregulates MMP-1, MMP-2 and MMP-13 expression. J Dermatol Sci. 2001 4;25(3):198-205. PMID: 11240267.
13. Ohno I, Ohtani H, Nitta Y, Suzuki J, Hoshi H, Honma M, et al. Eosinophils as a source of matrix metalloproteinase-9 in asthmatic airway inflammation. Am J Respir Cell Mol Biol. 1997 3;16(3):212-219. PMID: 9070604.
14. Okada S, Kita H, George TJ, Gleich GJ, Leiferman KM. Migration of eosinophils through basement membrane components in vitro: role of matrix metalloproteinase-9. Am J Respir Cell Mol Biol. 1997 10;17(4):519-528. PMID: 9376127.
15. Nakaya M, Dohi M, Okunishi K, Nakagome K, Tanaka R, Imamura M, et al. Prolonged allergen challenge in murine nasal allergic rhinitis: nasal airway remodeling and adaptation of nasal airway responsiveness. Laryngoscope. 2007 5;117(5):881-885. PMID: 17473688.
16. Namba M, Asano K, Kanai K, Kyo Y, Watanabe S, Hisamitsu T, et al. Suppression of matrix metalloproteinase production from nasal fibroblasts by fluticasone propionate in vitro. Acta Otolaryngol. 2004 10;124(8):964-969. PMID: 15513534.
17. Sakakura Y, Majima Y, Mitsui H, Inagaki M, Miyoshi Y. Absorption of various drugs through the rabbit's respiratory mucosa in vitro. Arch Otorhinolaryngol. 1983;238(1):87-96. PMID: 6349595.
Fig.┬Ā3Summary of histological evaluations. Light-microscopic images of H&E (A-F) and Alcian blue/periodic acid Schiff (G-I) in the control group (A, D, G). Allergic rhinitis group (B, E, H) and the group treated with doxycycline (C, F, I). Stars indicate vascular congestion, white arrows indicate goblet cells, and black arrows indicate eosinophilis. A-C: H&E,├Ś40; D-F: H&E,├Ś100; G-I, Alcian blue/periodic acid Schiff,├Ś40. 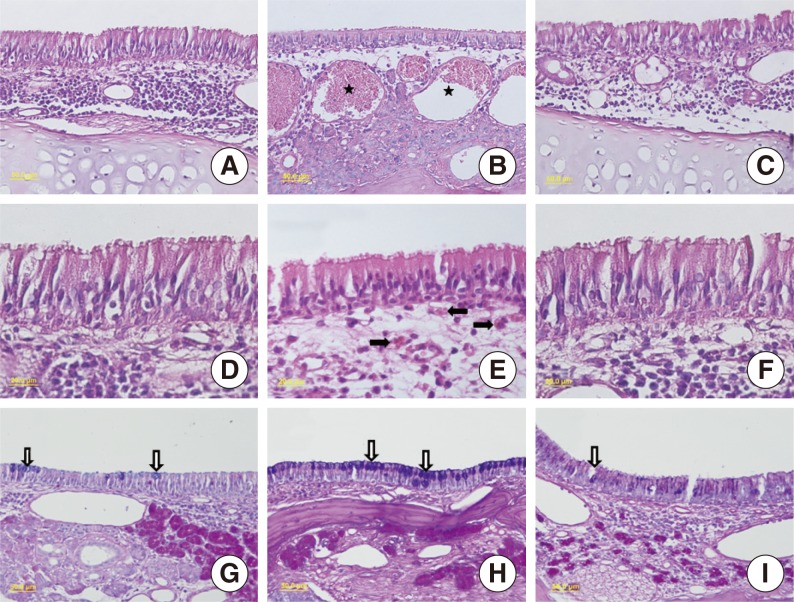
|
|
||||||||||||||||||||||||||||||||||||||||||||||