 |
 |
- Search
AbstractObjectivesPatients with smell loss after craniocerebral trauma are known to have some brain abnormalities, but there was no study to analyze the findings according to the time interval between injury and evaluation. We aimed to identify whether the time interval may influence on the findings in the brain.
MethodsMedical records of 19 patients with posttraumatic olfactory dysfunction were reviewed. All of them underwent a magnetic resonance imaging and olfactory function tests. The patients were divided into early (n=10) and delayed (n=9) groups according to the time interval.
ResultsMagnetic resonance imaging was taken at a mean time of 2.2 and 59.6 months after trauma in the early and delayed groups, respectively. Abnormal findings in the brain were found in 6 and 8 patients in the early and delayed groups, respectively. The olfactory bulb and orbitofrontal cortex were commonly affected olfactory pathways in both groups. In the early group, the abnormalities were brain tissue defect, hemorrhage, and focal edema whereas tissue defect was the only finding in the delayed group. In the early group, 5 of 6 patients with severe olfactory dysfunction showed brain abnormality while 1 of 4 patients with mild dysfunction had abnormality. In the delayed group, all the patients had severe dysfunction and 8 of 9 patients showed brain abnormality.
The incidence of olfactory dysfunction associated with head trauma varies from 5% to 25% and it is known to increase in proportion to the severity of injury [1,2,3]. Prior studies have noted that as many as 20% to 35% of head trauma victims with olfactory dysfunction experience improvement in 2-3 years [4,5]. Although specific mechanisms of olfactory loss have not been fully clarified, three pathophysiological mechanisms of traumatic olfactory dysfunction have been established thus far: shearing or tearing of the olfactory nerve fibers at the cribriform plate, mechanical obstruction of the sinonasal tract, and central brain lesions such as intracranial hemorrhage or contusion in the olfaction-related brain regions [3,6]. A disorganized appearance of the olfactory epithelia, a marked decrease in the number of ciliated olfactory receptor, and a lack of intact olfactory glomeruli in the olfactory bulb were identified in previous studies. However, histologic examination of the human olfactory apparatus is rarely possible [6,7]. Instead, several imaging studies such as computed tomography (CT), magnetic resonance imaging (MRI), single photon emission tomography (SPECT), and positron emission tomography (PET) have been employed to elucidate the pathophysiology of posttraumatic olfactory dysfunction [8,9,10,11,12]. MRI has been recognized to have advantages in investigating injuries to the brain soft tissue lesions due to high soft tissue resolution and the absence of the beam hardening artifact observed in CT [13]. Even though the patients with olfactory dysfunction after trauma are known to have some abnormalities, there was no study to analyze the findings according to the the time interval between injury and evaluation. This study is aimed to identify whether the time interval may influence on the findings in the brain.
The present study included 19 patients who visited the Smell Disorder Clinic in Seoul National University Bundang Hospital for posttraumatic olfactory dysfunction and underwent brain MRI as well as olfactory function tests. All the patients were examined by nasal endoscopy and showed patent passages in the bilateral olfactory clefts. The mean time interval between head injury and first evaluation of olfactory function was 31 months (range, 1 to 120 months). The patients were categorized into the early group (n=10) and delayed group (n=9). The early group underwent MRI within 3 months after head injury and the delayed group at least 6 months after the accident. When all the patients visited our clinic, no witnessed cognitive abnormality was reported by their family members. This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital.
The coronal images were obtained using 3-Tesla MR imaging system (Philips, Achieva, Best, The Netherlands) with a 1 mm thin slice from the cribriform plate to the sella turcica. The parameters were as follows: T1-weighted images (repetitive time [TR], 585; echo time [TE], 10; echo train length [ETL], 0; number of excitations [NSA], 2; field of view [FOV], 18 cm; matrix, 512├Ś512; slice thickness, 1 mm); T2-weighted images (TR, 3,500; TE, 120; ETL, 21; NSA, 3; FOV, 18 cm; matrix, 512├Ś512; thickness, 1 mm). On T1- and T2-weighted coronal images, the olfactory bulb was well visualized and additional T1- and T2-weighted axial images were used for the analysis of other anatomical structures such as the orbitofrontal cortex, prefrontal cortex, hippocampus, amygdala and temporal lobe. Images were evaluated by a radiologist, specialized in neuro-head and neck radiology, who was blinded to the olfactory function test scores of each patient.
The Korean version of Sniffin' sticks (KVSS) identification test was used to assess olfactory function for each patient [14]. The KVSS identification test is a modification of Sniffin' sticks for Koreans which is well validated and widely used in Korea [14,15,16]. It contains 16 odorants familiar to Koreans. Anosmia was defined as scores 0 to 3, severe hyposmia as scores 4 to 7, mild to moderate as scores 8 to 12, and normosmia as scores 13 to 16. Anosmia and severe hyposmia were considered as severe olfactory dysfunction and mild to moderate hyposmia as mild olfactory dysfunction.
Mann-Whitney U-test was performed to compare the mean age and Fisher exact test to compare gender distribution. The association of the severity of olfactory dysfunction and the presence of abnormalities in MRI was also evaluated by Fisher exact test. All statistical analyses were conducted using the SigmaStat for Windows SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). The P-value of <0.05 was considered statistically significant.
The early group included 6 males and 4 females. Their mean age was 40.5 years (range, 22 to 59 years). The delayed group included 7 males and 2 females. Their mean age was 40.1 years (range, 19 to 54 years). The gender distribution and mean age were not different between the two groups (P=0.63 and P=0.96, respectively). There were brain abnormalities in 14 of the 19 patients (73.7%) included in the present study.
In the early group, patients underwent MRI at a mean time of 2.2 months (range, 1 to 3 months) after head injury. The causes of injury were motor vehicle accident (MVA) (n=6) and falldown (n=4). Six of 10 patients in the early group exhibited abnormal findings in the brain MRI. The brain abnormalities were brain tissue defects, hemorrhage and focal edema (Table 1). Extensive brain tissue defects were found in 4 patients and all of them exhibited severe olfactory dysfunction. Two of them also had intracranial hemorrhage such as intraparenchymal, subdural and subarachnoid hemorrhage (Fig. 1A) at the same time. Focal edema was found in 2 patients. One patient with edema alone had mild olfactory dysfunction (Fig. 1B) while the other with both edema and hemorrhage demonstrated severe olfactory dysfunction. The remaining four patients showed normal MR findings.
In the delayed group, MRI was taken at a mean time of 59.6 months (range, 8 to 120 months) after trauma. The causes of trauma were MVA (n=4), assault (n=4), and falldown (n=1). All the patients in the delayed group showed severe olfactory dysfunction. Eight of 9 patients in the delayed group exhibited abnormal findings in the brain MRI. Brain tissue defect was the only finding in the delayed group and it was observed in 8 patients (Table 2). Seven patients showed bilateral brain tissue defects. The area of brain tissue defect was manifested as encephalomalacic changes and the empty regions were filled with cerebrospinal fluid (Fig. 1C). One patient showed unilateral brain tissue defect and normal MRI finding was present in one patient.
The brain regions showing abnormal MR findings in the early group were orbitofrontal cortex (n=6, 100%), olfactory bulb (n=4, 66.7%), temporal lobe (n=4, 66.7%), prefrontal cortex (n=3, 50.0%), and hippocampus or amygdale (n=2, 33.3%). In the delayed group, they were olfactory bulb (n=8, 100%), orbitofrontal cortex (n=7, 87.5%), prefrontal cortex (n=4, 50.0%), temporal lobe (n=4, 50.0%), and hippocampus or amygdale (n=3, 37.5%). Of the 15 patients who had severe olfactory dysfunction in both groups, 13 patients (86.7%) showed abnormal MR findings. On the contrary, only one of the four patients (25%) with mild olfactory dysfunction demonstrated abnormal MR findings (P=0.037).
Several neuropsychiatric disorders such as Alzheimer disease, Parkinson's disease, Korsakoff's psychosis, and schizophrenia are known to be associated with olfactory dysfunction and abnormalities can be identified in brain MRI in several areas associated with olfaction such as the prefrontal lobe, temporal lobe, hippocampus and thalamus [13]. Unlike these diseases, MRI is not routinely performed in patients with olfactory loss associated with head trauma. Therefore, there were a limited number of studies concerning the brain findings in posttraumatic olfactory dysfunction. A previous pinoneering study showed that 88% of patients with posttraumatic olfactory dysfunction had brain abnormality in the areas associated with sense of smell [12]. In the above-mentioned study, the brain abnormality was mainly found in the olfactory bulbs and tract and inferior frontal lobes and there was no significant correlation of the injured areas with the olfactory test scores [12]. Our study also showed that brain abnormality was observed about in three fourths of the patients and that the olfactory bulb and orbitofrontal cortex were predominantly involved brain regions.
To our best knowledge, there have been no studies to investigate the abnormal findings in the brain according to the the time interval between head trauma and evaluation of brain MRI. In spite of a comprehensive review of the literature, only a single case report was found with respect to the early brain changes in patients with posttraumatic olfactory dysfunction [9]. Therein, MRI was evaluated and the main lesion was hemorrhage. Our study also showed that hemorrhage was the brain finding observed only in the early group. The early group also had tissue defect and focal edema in the brain. On the other hand, in the delayed group, brain tissue defect was the only finding. Interestingly, hemorrhage as well as brain tissue defect was closely associated with severe olfactory dysfunction. Hemorrhage serves as an iron source, which favors the production of hydroxyl radicals via the Haber-Weiss reaction of the xanthine oxidase pathway [17]. Therefore, it is assumed that while hemorrhage in the early period resolves over time, oxidative injury potentiated by hemorrhage might aggravate the damage of the olfactory brain regions, resulting in extensive encephalomalacic changes. Accordingly, hemorrhage might be associated with severe olfactory dysfunction. On the contrary, a single patient with focal edema alone showed mild olfactory dysfunction. Because of the lack of the number of patients, however, further study is needed in order to confirm the significance of focal brain edema in the areas associated with olfaction.
As mentioned above, shearing or tearing of olfactory nerve fibers at the cribriform plate is recognized as one of the mechanisms of posttraumatic olfactory dysfunction. Three out of four patients with mild olfactory dysfunction showed no abnormalities in the brain, probably due to this mechanism. In contrast, only two of 15 patients with severe olfactory dysfunction demonstrated no abnormalities in MRI. In other words, most severe olfactory dysfunction might be associated not only with shear or tearing of the olfactory nerve fibers, but also with other brain abnormalities which can be identified in the brain MRI.
The limitation of this study is a small population of patients. Additionally, given that patients with mild smell loss might recover over time, with low chances to visit clinics, there might be a selection bias. For clearer conclusions, a prospective cohort study will be needed.
In conclusion, during the early posttraumatic period, various findings such as focal edema, hemorrhage, and brain tissue defects were visualized whereas brain tissue defect was the only finding when the evaluation of the brain was delayed. Despite some limitations of this study, we demonstrated abnormal brain findings in most of the patients and it is suggested that brain abnormality might be different according to the timing of evaluation. Additionally, there seems to be an association between the severity of olfactory dysfunction and abnormal findings identified by MRI.
References2. Yousem DM, Geckle RJ, Bilker WB, Kroger H, Doty RL. Posttraumatic smell loss: relationship of psychophysical tests and volumes of the olfactory bulbs and tracts and the temporal lobes. Acad Radiol. 1999 5;6(5):264-272. PMID: 10228615.
3. Reiter ER, DiNardo LJ, Costanzo RM. Effects of head injury on olfaction and taste. Otolaryngol Clin North Am. 2004 12;37(6):1167-1184. PMID: 15563909.
4. Duncan HJ, Seiden AM. Long-term follow-up of olfactory loss secondary to head trauma and upper respiratory tract infection. Arch Otolaryngol Head Neck Surg. 1995 10;121(10):1183-1187. PMID: 7546588.
5. Fujii M, Fukazawa K, Takayasu S, Sakagami M. Olfactory dysfunction in patients with head trauma. Auris Nasus Larynx. 2002 1;29(1):35-40. PMID: 11772488.
6. Kern RC, Quinn B, Rosseau G, Farbman AI. Post-traumatic olfactory dysfunction. Laryngoscope. 2000 12;110(12):2106-2109. PMID: 11129030.
7. Moran DT, Jafek BW, Rowley JC 3rd, Eller PM. Electron microscopy of olfactory epithelia in two patients with anosmia. Arch Otolaryngol. 1985 2;111(2):122-126. PMID: 3977726.
8. Duprez TP, Rombaux P. Imaging the olfactory tract (cranial nerve #1). Eur J Radiol. 2010 5;74(2):288-298. PMID: 20303227.
9. Wise JB, Moonis G, Mirza N. Magnetic resonance imaging findings in the evaluation of traumatic anosmia. Ann Otol Rhinol Laryngol. 2006 2;115(2):124-127. PMID: 16514795.
10. Eftekhari M, Assadi M, Kazemi M, Saghari M, Esfahani AF, Sichani BF, et al. A preliminary study of neuroSPECT evaluation of patients with post-traumatic smell impairment. BMC Nucl Med. 2005 11;5:6PMID: 16313675.
11. Varney NR, Pinkston JB, Wu JC. Quantitative PET findings in patients with posttraumatic anosmia. J Head Trauma Rehabil. 2001 6;16(3):253-259. PMID: 11346447.
12. Yousem DM, Geckle RJ, Bilker WB, McKeown DA, Doty RL. Posttraumatic olfactory dysfunction: MR and clinical evaluation. AJNR Am J Neuroradiol. 1996;Jun-Jul;17(6):1171-1179. PMID: 8791933.
13. Li C, Yousem DM, Doty RL, Kennedy DW. Neuroimaging in patients with olfactory dysfunction. AJR Am J Roentgenol. 1994 2;162(2):411-418. PMID: 8310937.
14. Hong SC, Yoo YS, Kim ES, Kim SC, Park SH, Kim JK, et al. Development of KVSS test (Korean version of Sniffin' sticks test). Korean J Otolaryngol-Head Neck Surg. 1999 7;42(7):855-860.
15. An SY, Kong IG, Lee CH, Kim JW. Analysis of the correct-answer rate of the odor identification test in KVSS (Korean version of Sniffin' sticks) test. Korean J Otorhinolaryngol-Head Neck Surg. 2007 12;50(12):1109-1113.
16. Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. 'Sniffin' sticks': olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses. 1997 2;22(1):39-52. PMID: 9056084.
17. Sande A, West C. Traumatic brain injury: a review of pathophysiology and management. J Vet Emerg Crit Care (San Antonio). 2010 4;20(2):177-190. PMID: 20487246.
Fig.┬Ā1Representative abnormal magnetic resonance findings. (A) Subarachnoid hemorrhage in the bilateral olfactory bulb and orbitofrontal cortex, (B) focal edema in the bilateral orbitofrontal cortices, (C) a large defect in the right orbitofrontal cortex filled with cerebrospinal fluid. 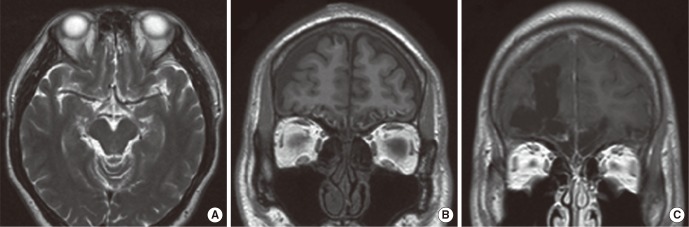
Table┬Ā1MR findings and olfactory dysfunction in the early group 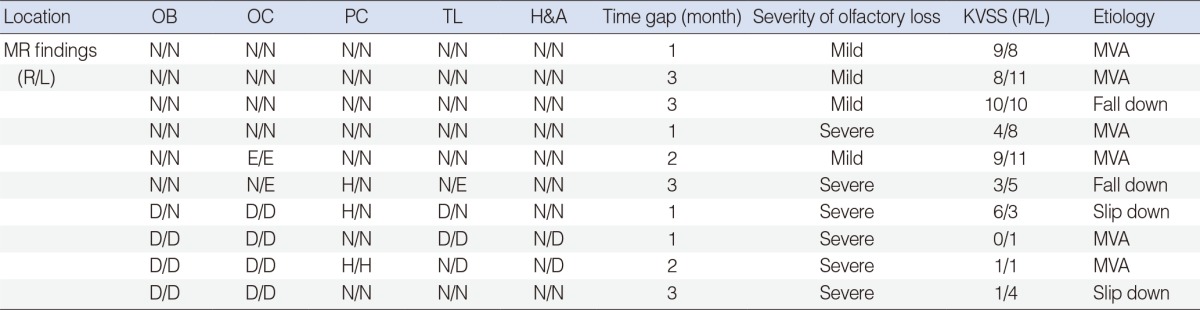 Time gap means the time interval between head injury and olfactory function evaluation. The degree of 4 hyposmic patients was mild to moderate. MR, magnetic resonance; OB, olfactory bulb; OC, orbitofrontal cortex; PC, prefrontal cortex; TL, temporal lobe; H&A, hippocampus and amygdale; KVSS, Korean version of Sniffin' sticks; R, right; L, left; N, normal finding; MVA, motor vehicle accident; E, edema; H, hemorrhage; D, defect. Table┬Ā2MR findings and olfactory dysfunction in the delayed group 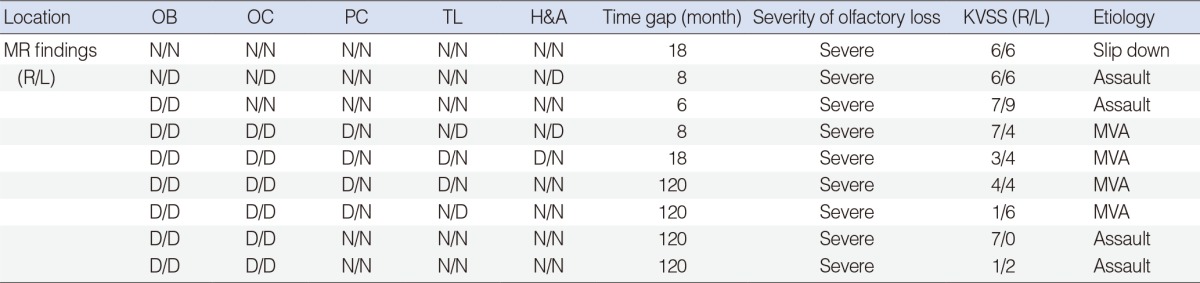 Time gap means the time interval between head injury and olfactory function evaluation. MR, magnetic resonance; OB, olfactory bulb; OC, orbitofrontal cortex; PC, prefrontal cortex; TL, temporal lobe; H&A, hippocampus and amygdale; KVSS, Korean version of Sniffin' sticks; R, right; L, left; N, normal finding; D, defect; MVA, motor vehicle accident. |
|
||||||||||||||||||||||||||||||||||||||||||







