INTRODUCTION
Mucus secretion in the human airway plays an important role in protection of the lungs and upper airway tracts from the external environment. Mucus entraps inhaled foreign particles and bacteria, and clears them from the airway by ciliary movement [1]. In inflammatory airway diseases, excessive mucus secretion leads to increases in the morbidity and mortality of the affected patients [2]. Mucins, which are highly glycosylated proteins, are the major components of mucus. The two types of mucins in human airway tracts are secreted and membrane-bound forms. MUC5AC, MUC5B, and MUC8 are representative secretory mucins [3]. These mucins are regulated by many pathophysiological mediators. Toll like receptor (TLR)-mediated airway inflammatory action has recently been studied [4,5]. TLR induces an immune response through activation of mitogen-activated protein kinase (MAPK) and nuclear factor kappa-right-chain-enhancer of activated B cells (NF-κB) in human airway epithelial cells [6]. These findings have implicated TLR as a potential new therapeutic target in airway inflammatory diseases.
Delphinidin, one of the anthocyanidins, is found in many pigmented fruits and vegetables. In long overdue studies, it has demonstrated beneficial effects on cardiovascular disease and cancers by exhibiting antioxidant, antiangiogenic, and anti-cancer properties [7,8,9,10]. Recent study has reported that delphinidin protected small intestine against inflammation induced by ischemia-reperfusion [11]. However, the effects of delphinidin on mucin secretion in inflammatory airway epithelial cells have not yet been reported. Therefore, this study aimed to evaluate the effect of delphinidin on mucin expression and its signaling pathways in human airway epithelial cells.
MATERIALS AND METHODS
Materials
Lipopolysaccharide (LPS) and delphinidin were obtained from Sigma-Aldrich (St. Louis, MO, USA). Mucin-producing human NCI-H292 airway epithelial cells were obtained from the American Type Culture Collection (Manassas, VA, USA). RPMI 1640 medium and Trizol were obtained from Invitrogen (Carlsbad, CA, USA). Reverse transcriptase-polymerase chain reaction (RT-PCR) kits were obtained from Applied Biosystems (Foster City, CA, USA). Real-time PCR kits were obtained from Roche Applied Science (Mannheim, Germany). Fetal bovine serum (FBS) was obtained from Hyclone Laboratories (Logan, UT, USA). Enhanced chemiluminescence reagents were obtained from Perkin Elmer Life Sciences (Boston, MA, USA). MUC8 (sc-16920), MUC5B (sc-23024), anti-goat or anti-mouse horseradish peroxidase (HRP)-conjugated secondary antibodies, and anti-rabbit HRP-conjugated secondary antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Extracellular signal related kinase (ERK)1/2, phospho-ERK1/2, p38, and phospho-p38 were purchased from Cell Signaling Technology (Danvers, MA, USA). Predesigned small interfering RNA (siRNA) targeting TLR4 and negative control siRNA were purchased from Invitrogen Corporation (Carlsbad, CA, USA). For the primary culture, nasal mucosa was obtained from normal inferior turbinate samples from 10 patients undergoing augmentation rhinoplasty who had no personal or family history of allergy, and who had negative results on skin-prick tests to 20 common airborne allergens and on multiple simultaneous allergen tests. This study was approved by the institutional review board for human studies at the Yeungnam University Medical Center and written informed consent was obtained from each patient.
Cell culture and treatment
Human NCI-H292 airway epithelial cells were cultured in RPMI 1640 medium supplemented with 2 mM L-glutamine, 100 U/mL penicillin, 100 µg/mL streptomycin, and 10% FBS. The cells were grown at 37℃ in 5% CO2 fully humidified air and subcultured twice weekly. The cells were seeded in wells of a 6-well plate at 1×105 cells/well. When the cultures had become confluent, the cells were incubated in RPMI 1640 medium containing 0.5% FBS for 24 hours. The cells were then rinsed with serum-free RPMI 1640 medium and exposed to the indicated concentrations of delphindin. For the primary culture of human nasal epithelial cells, the nasal mucosal tissue was washed with phosphate-buffered saline (PBS) and immersed in dispase (Boehringer Mannheim Biochemica, Mannheim, Germany) for 90 minutes. The tissue was scraped off the surface of the nasal mucosa using a scalpel, and added to 1% PBS and filtered through a mesh. The cells were seeded in a 24-well plate at 2.5×105 cells/well and were then incubated with EpiLife medium and human keratinocyte growth supplement (5 mL/500 mL of medium). When the cultures had become confluent, the cells were exposed to the indicated concentrations of delphinidin.
To investigate mucin gene expression, human NCI-H292 airway epithelial cells and human nasal epithelial cells were pretreated with LPS for 1 hour before exposure to the indicated concentrations of delphinidin. For the controls, human NCI-H292 airway epithelial cells and human nasal epithelial cells were incubated with the medium alone for the same amount of time.
RT-PCR analysis of TLR4, MUC8, and MUC5B mRNA expression
Total RNAs from LPS treated cultured cells were prepared using the available reagent according to the manufacturer's instructions (Applied Biosystems). Each sample was reverse transcribed into cDNA using the GeneAmp RNA PCR Core Kit (Applied Biosystems). The primer sequences used in the PCR were 5'-CAC ATC CAC CCT TCC AAC-3' (sense) and 5'-GGC TCA TTG TCG TCT CTG-3' (antisense) for MUC5B, and 5'-ACA GGG TTT CTC CTC ATT G-3' (sense) and 5'-CGT TTA TTC CAG CAC TGT TC-3' (antisense) for MUC8. PCR for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was performed on each individual sample as a positive control. The primer sequence of GAPDH used in the PCR was 5'-CCT CCA AGG AGT AAG ACC CC-3' (sense) and 5'-AGG GGT CTA CAT GGC AAC TG-3' (antisense). PCR products were electrophoresed on a 2% agarose gel, stained with ethidium bromide, and visualized by ultraviolet fluorescence. Semiquantitative analysis of the RT-PCR product was performed on the scanned gel images, and the intensity of the PCR product was measured using commercially available imaging software (Scion, Frederick, MD, USA). The relative intensity of individual bands on the gel image was determined as the ratio of intensities of TLR4, MUC8, and MUC5B to the intensity of GAPDH.
Real-time PCR analysis of MUC8 and MU5B mRNA expression
Real-time PCR was performed using the LC Fast Start DNA Master SYBR Green kit using 0.5 µL of cDNA, corresponding to 25 ng of total RNA in a 10 µL final volume, 2.5 mM MgCl2 and 0.5 µM of each primer (final concentration). Quantitative PCR was performed using a LightCycler for 45 cycles at 95℃ for 10 seconds, specific annealing temperature for 5 seconds and 72℃ for 10 seconds. Data were normalized to GAPDH. Amplification specificity was evaluated using melting curve, following the manufacturer's instructions (Roche Applied Science).
ELISA analysis of MUC5B and MUC8 protein
Protein levels for MUC5B and MUC8 were determined by enzyme-linked immunosorbent assay (ELISA). Sample of cell lysates from NCI-H292 cells was incubated at 40℃ in a 96-well plate until dry. Plates were then washed three times with PBS, blocked with 2% bovine serum albumin for 1 hour at room temperature, washed again three times with PBS, and incubated with primary antibody for MUC8 or MUC5B diluted at 1:200 with PBS containing 0.05% tween 20 for 1 hour. Wells were treated by dispensation of HRP-conjugated secondary antibody into each well. After 4 hours, color was developed using 3,3', 5,5'-tetramethylbenzidine peroxidase solution and stopped with 2N-H2SO4. Optical density measurements were performed using an EL800 ELISA reader (BIO-TEK Instruments, Winooski, VT, USA) at 450 nm.
Western blot analysis of ERK1/2 and p38 MAPK phosphorylation
NCI-H292 cells were seeded in a 6-well plate and treated with delphinidin. Cells were exposed to trypsin, and formed into pellets at 700 g at 4℃; pellets were resuspended in lysis buffer. The preparation was then clarified by centrifugation, and the supernatant was saved as a whole-cell lysate. Proteins (20 µg) were separated using 10% reducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electroblotted onto a nitrocellulose membrane. The membrane was blocked with 5% nonfat dry milk followed by incubation with the indicated primary antibody of ERK1/2 or p38 for 4 hours. Subsequently, the membrane was incubated for 1 hour with secondary antibody of ERK1/2 or p38 conjugated to HRP, and developed using an enhanced chemiluminescence kit (Perkin Elmer Life Sciences) Bands were detected after exposure to X-ray film for 10 seconds.
Cell transfection with small interfering RNA (siRNA) for TLR4
To verify involvement of TLR4 in LPS-induced MUC8 and MU5B expression, the cells were transfected with TLR4 siRNA. Transfection was performed according to the manufacturer's protocol (Invitrogen). Briefly, NCI-H292 cells were seeded in wells of a 6-well plate at 1×105 cells/well and incubated in RPMI 1640 medium. When the cells had become confluent, OPTI-MEM I Reduced Serum Medium was added. Then, TLR4 siRNA and Lipofectamine 2000 were incubated together in OPTI-MEM I Reduced Serum Medium to form a TLR4 siRNA-Lipofectamine complex. The TLR4 siRNA-Lipofectamine complex-containing medium was added to each well containing cells to a final TLR4 siRNA concentration of 20 nM. After 24 hours of transfection with TLR4 siRNA, the cells were exposed to the indicated concentration of LPS and then harvested for RT-PCR analysis of MUC8 and MUC5B mRNA expression. The same procedure was performed with negative control siRNA (treated with Lipofectamine 2000 only). The transfection rate of TLR4 siRNA was verified to be over 90% in NCI-H292 cells. For the controls, NCI-H292 cells were incubated with the medium alone for the same time (not treated).
RESULTS
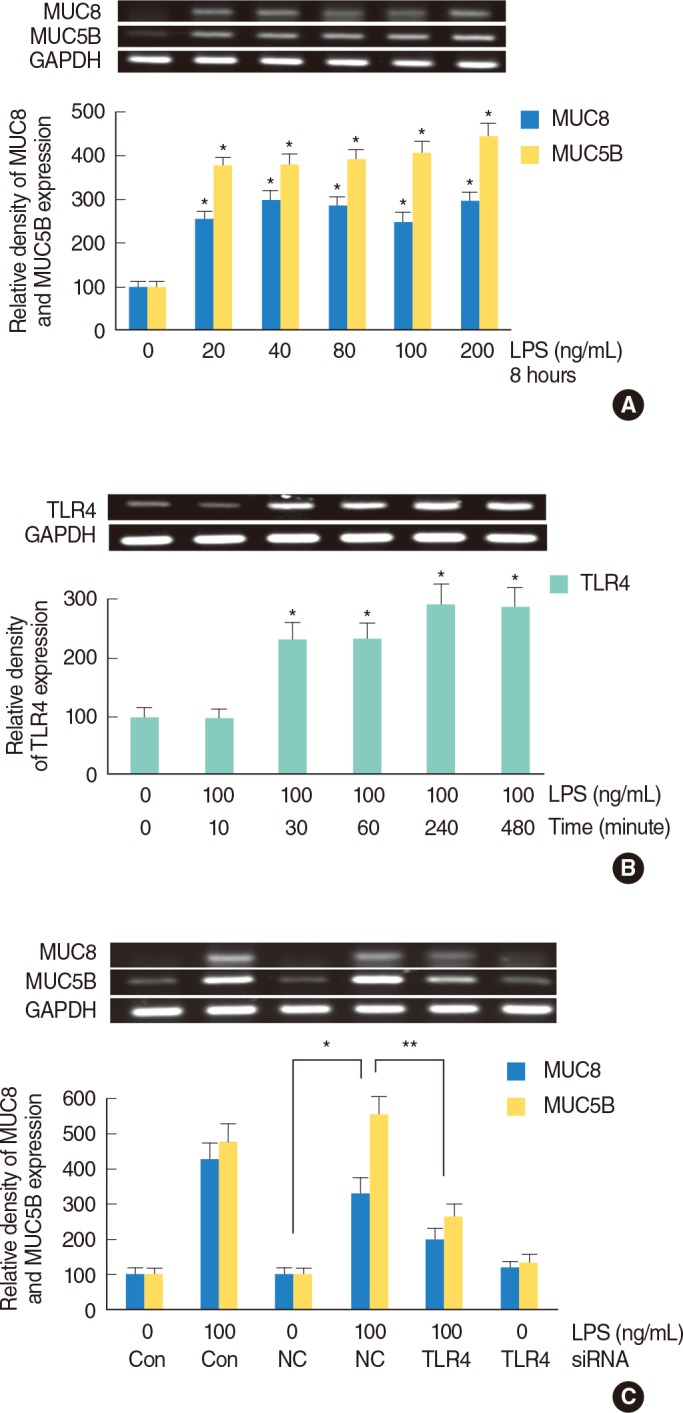
LPS-induced TLR4, MUC8, and MUC5B expression in NCI-H292 cells
NCI-H292 cells were incubated with LPS (0, 20, 40, 80, 100, and 200 ng/mL) for 8 hours and MUC8 and MUC5B mRNA expression levels were analyzed by RT-PCR. MUC8 and MUC5B mRNA expressions were significantly increased by treatment with all dosages of LPS (Fig. 1A). MUC8 and MUC5B glycoprotein levels were also significantly increased by LPS: MUC8 and MUC5B glycoprotein level was peaked at 100 ng/mL of LPS (data not shown). Through the results of MUC8 and MUC5B expression, the dose of 100 ng/mL of LPS was used for the definite inflammatory stimulation on human airway epithelial cells. To evaluate the effect of LPS on TLR4 mRNA expression, NCI-H292 cells were incubated with LPS (100 ng/mL) for different lengths of time (0, 10, 30, 60, 240, and 480 minutes). The result of RT-PCR showed that TLR4 mRNA expression was significantly increased by LPS in a time-dependent manner (Fig. 1B). To identify the relationship between TLR4 expression and LPS-induced MUC8 and MUC5B expression, study of TLR4-siRNA was performed. The result of RT-PCR showed that knockdown of TLR4 by TLR4-siRNA significantly blocked LPS-induced MUC8 and MUC5B mRNA expression (Fig. 1C).
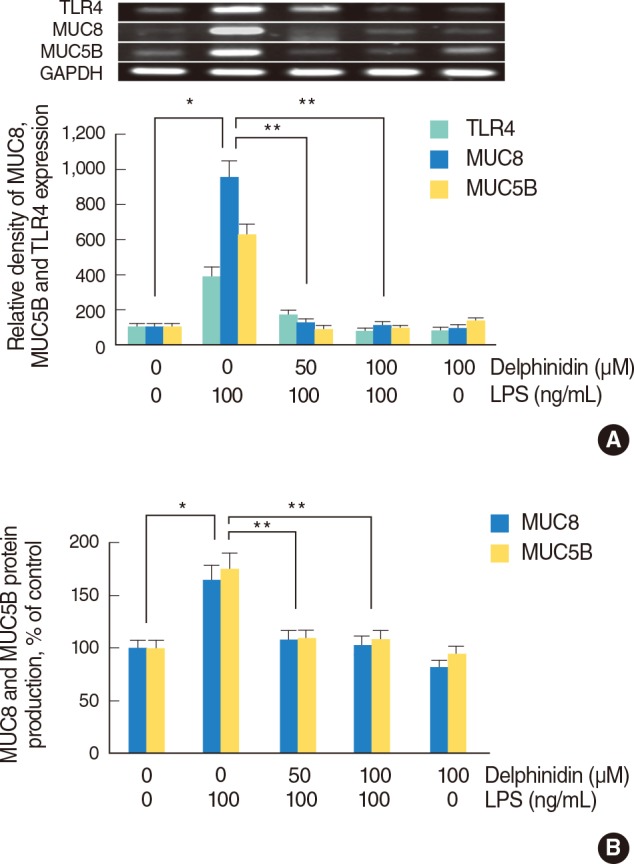
The effect of delphinidin on LPS-induced TLR4, MUC8, and MUC5B expression in NCI-H292 cells
To investigate the effect of delphinidin on LPS-induced TLR4, MUC8, and MUC5B expression, NCI-H292 cells were pretreated with the different doses of delphinidin (0, 50, and 100 µM) for 1 hour before being incubated with LPS (100 ng/mL) for 8 hours [12]. The results of RT-PCR and ELISA showed that LPS induced MUC5B and MUC8 mRNA expression and protein productions, which were significantly decreased by delphinidin, and LPS-induced TLR4 mRNA expression was markedly decreased by delphinidin (Fig. 2A, B).
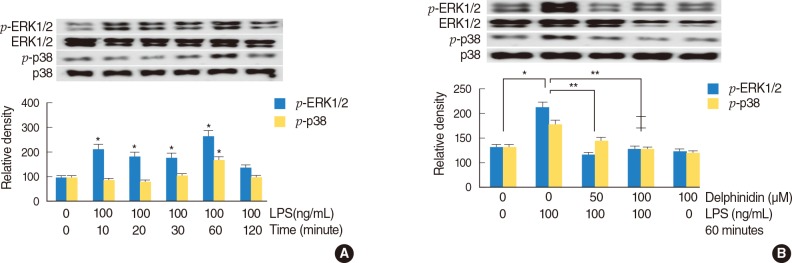
The effect of delphinidin on LPS-induced phosphorylation of ERK1/2 and p38 MAPK in NCI-H292 cells
To investigate the effect of LPS on phosphorylation of ERK1/2 and p38 MAPK, NCI-H292 cells were incubated with LPS (100 ng/mL) for different lengths of time (0, 10, 20, 30, 60, and 120 minutes). Western blot was performed for analysis of phosphorylation of ERK1/2 and p38 MAPK. The result showed that phosphorylation of ERK1/2 and p38 MAPK were significantly increased by LPS at a time of 60 minutes (Fig. 3A). To investigate the effect of delphinidin on LPS-induced phosphorylation of ERK1/2 and p38 MAPK, NCI-H292 cells were pretreated with delphinidin (0, 50, and 100 µM) for 60 minutes before being incubated with LPS (100 ng/mL) for 60 minutes. The result of Western blot showed that delphinidin significantly decreased LPS-induced phosphorylation of ERK1/2 and p38 MAPK (Fig. 3B).
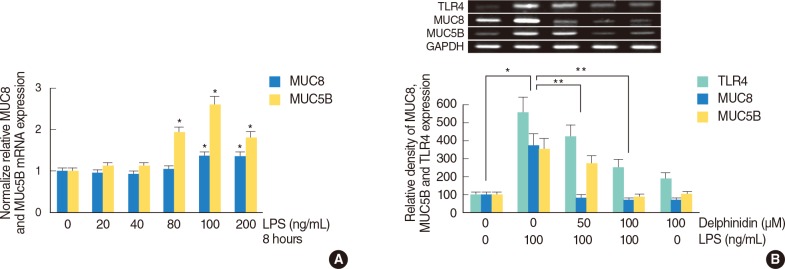
The effect of delphinidin on LPS-induced TLR4, MUC8 and MUC5B expression in human nasal epithelial cells
To evaluate the effect of LPS on MUC8 and MUC5B expression in primary cultures of human nasal epithelial cells, the cells were incubated with LPS (0, 20, 40, 80, 100, and 200 ng/mL) for 8 hours and real time PCR was performed for analysis of MUC8 and MUC5B mRNA expression. The result showed that MUC8 and MUC5B mRNA expression were significantly increased by LPS; the expression peaked at 100 ng/mL of LPS (Fig. 4A). To evaluate the effect of delphinidin on LPS-induced MUC8 and MUC5B expression in primary cultures of human nasal epithelial cells, the cells were pretreated with the different doses of delphinidin (0, 50, and 100 µM) for 1 hour before being incubated with LPS (100 ng/mL) for 8 hours. The results of RT-PCR showed that delphinidin significantly attenuated LPS-induced TLR4, MUC8, and MUC5B mRNA expression (Fig. 4B).
DISCUSSION
Chronic inflammatory airway diseases, such as chronic rhinosinusitis, chronic obstructive pulmonary diseases, and asthma were difficult to control due to various factors. Among a variety of factors, excessive mucus production is a critical factor associated with inflammation by infection of microorganisms [13]. However, the mechanism of mucus overproduction has not yet been completely elucidated.
Mucins, approximately 2% of mucus, are responsible for the physical and biological properties of mucus. The predominant mucins present in inflammatory airway disease are MUC4, MUC5AC, MUC5B, and MUC8, which are induced by a variety of inflammatory mediators such as LPS, interleukins, leukotrienes, and several chemicals [13,14,15].
LPS is the major outer surface membrane component present in almost all Gram-negative bacteria and is a strong stimulator of inflammation. LPS binds mainly to TLR4 receptor. Activation of TLR4 by LPS induces activation of the NF-κB and finally results in the release of proinflammatory cytokines [16]. LPS also induces activation of MAPKs through both TLR4-mediated signaling pathways: the myeloid differentiation primary response gene 88 (MyD88)-dependent and MyD88-independent pathways [17]. Therefore several studies have reported that LPS induces variable mucin expression via TLR4 and MAPKs signaling pathways [13,18], and this study focused on LPS, MUC8, MUC5B, TLR4, ERK1/2 MAPK, and p38 MAPK. The results of this study showed that LPS induced MUC8 and MUC5B expression and LPS induced TLR4 expression and phosphorylation of ERK1/2 and p38 MAPK. In addition, TLR4 siRNA-mediated knockdown of TLR4 significantly blocked LPS-induced MUC8 and MUC5B expression. These findings suggest that TLR4, ERK1/2 MAPK, and p38 MAPK play important roles in LPS-induced MUC8 and MUC5B expression in human airway epithelial cells.
Among anthocyanidins, delphinidin appears to possess strong beneficial effects, including antitumoral, antimutagenic, anticarcinogenic, and antiangigenic activities [7,8,9]. Recently, there is increasing evidence indicating a possible association of delphinidin with several processes involved in several inflammatory diseases [10,11,12,19]. In gastric epithelial cells, anthocyanins decrease Helicobacter pylori-induced reactive oxygen species (ROS) enhancement, nitric oxide synthases expression, and cyclooxygenase-2 (COX-2) expression. In addition, anthocyanins also inhibit phosphorylation of MAPK [19]. In LPS-activated murine macrophage cells, anthocyanidins inhibit LPS-induced COX-2 expression by blocking MAPK-mediated pathways with the attendant activation of NF-κB, activator protein-1 (AP-1) [12]. However, the effects and precise mechanisms of delphinidin on human airway epithelial cells have not been studied.
Therefore, this study focused on the potential anti-inflammatory property of delphinidin in inflammatory airway epithelial cells. The results of this study showed that delphinidin significantly attenuated not only LPS-induced TLR4, MUC8, and MUC5B expression, but also LPS-induced phosphorylation of ERK1/2 and p38 MAPK. These findings suggest that delphinidin inhibits LPS-induced MUC8 and MUC5B expression by attenuating of TLR4 expression and inhibiting the activation of ERK1/2 and p38 MAPK in human airway epithelial cells. However, the precise mechanism of the signaling pathway is still unclear and conduct of further studies is needed.
In conclusion, the results of this study demonstrate for the first time that delphinidin attenuates LPS-induced MUC8 and MUC5B expression through TLR4-mediated ERK1/2 and p38 MAPK signaling pathway in human airway epithelial cells. Therefore, delphinidin may have a protective effect on inflammatory airway diseases through regulation of mucin overproduction, which could be a novel therapeutic agent for control of inflammatory airway diseases.