 |
 |
- Search
AbstractObjectivesTo examine the relationship between speech intelligibilities among the similar level of hearing loss and threshold elevation of the auditory brainstem response (ABR).
MethodsThe relationship between maximum speech intelligibilities among similar levels of hearing loss and relative threshold elevation of the click-evoked ABR (ABR threshold - pure tone average at 2,000 and 4,000 Hz) was retrospectively reviewed in patients with sensorineural hearing loss (SNHL) other than apparent retrocochlear lesions as auditory neuropathy, vestibular schwannoma and the other brain lesions.
Patients with sensorineural hearing loss (SNHL) tend to suffer deterioration of speech intelligibility with progressive elevation of their hearing threshold [12]. However, speech intelligibility often varies among such patients with similar levels of hearing loss (HL). SNHL can be caused by many types of cochlear and/or retrocochlear pathologies, so that the deterioration of speech intelligibility may depend on the type of pathology. For example, speech intelligibilities tend to be worse in patients with retrocochlear lesions than in patients with cochlear lesions among subjects with similar hearing thresholds [3456]. Recent findings of auditory neuropathy have suggested that the involvement of inner hair cells could manifest as the same types of HL as retrocochlear HL, even as the HL due to impairment within the cochlea [7891011]. In other words, one possible explanation for the variation in speech intelligibilities among patients with similar levels of SNHL may be the different degree of involvement of the inner hair cells and/or cochlear nerves in addition to the outer hair cells. On the other hand, the auditory brainstem response (ABR) is very sensitive to the many types of pathology causing retrocochlear type HL, such as vestibular schwannoma, auditory neuropathy, multiple sclerosis, etc. [12131415161718]. Therefore, speech intelligibility should correlate with deterioration of the ABR if the variation in speech intelligibility is related to the severity of neural (cochlear nerve) and/or inner hair cell pathology in patients with SNHL. To test this hypothesis, the present study retrospectively examined the relationships between speech intelligibilities among patients with similar levels of SNHL and threshold elevation of the ABR in patients with SNHL other than apparent retrocochlear lesions as auditory neuropathy, vestibular schwannoma and the other brain lesions.
The present study was based on the data of 186 ears from 99 patients (50 males and 49 females; mean, 56.3±13.8 years; range, 23 to 80 years) who underwent speech audiometry as well as ABR measurement for the assessment of hearing difficulty in the Department of Otolaryngology-Head and Neck Surgery, Tohoku University Hospital since 2010. From this database, data fulfilling the following two criteria were selected: four-frequency average of pure tone air-conduction thresholds at 500, 1,000, 2,000, and 4,000 Hz (four-frequency pure tone average [PTA]) was worse than 25-dB HL; and absence of apparent retrocochlear pathology (auditory neuropathy, vestibular schwannoma, and the other brain lesions, etc.) and/or functional HL, based on the routine clinical examinations including the speech audiometry, Békésy audiometry, ABR, distortion product otoacoustic emissions (DPOAEs) and magnetic resonance imaging. Finally, 66 ears of 47 patients (24 males and 23 females; mean, 59.2±14.9 years; range, 25 to 80 years) were selected for analysis in the present study. No patient had any apparent abnormality in middle ear function based on the routine audiometry (air and bone conduction) and otoscopic findings.
The present study was approved by the Ethical Committee of the Tohoku University Graduate School of Medicine. All parts of the present study were performed in accordance with the guidelines of the Declaration of Helsinki.
Speech audiometry was conducted according to the standard method advocated by the Japan Audiological Society. A list of 20 Japanese monosyllables, compiled by the Japan Audiological Society and referred to as List 67-S, was used as the stimuli for speech audiometry. The level of the speech stimuli was elevated in 10-dB steps until the maximum percentage of correct answers was obtained at a sound level not exceeding 100 dB. The order of test stimuli for each sound level was randomly changed. Speech intelligibility was assessed by means of performance (percentage correct)-intensity (P-I) curves. The effects of HL on the P-I curves could be assessed by speech recognition threshold (50% correct level) as well as maximum speech intelligibility. However, speech recognition threshold could not be assessed in patients with maximum speech intelligibility of less than 50%, so the maximum speech intelligibility was assessed in such patients.
ABRs were recorded using a silver electrode placed on the mastoid and referred to the vertex. Measurements were conducted in a sound-proof room. Click stimuli were presented through an ear receiver at 10 Hz. Click phases were reversed alternately. The responses to 1,000 stimuli were filtered with a band pass filter of 50-3,000 Hz, then amplified and averaged using a signal processor (Neuropack S1 MEB 9402, Nihon Kohden, Tokyo, Japan). The level of click stimuli was usually decreased in 10-dB steps from 105-dB nHL until the responses were visually undetectable. The visual detection threshold was defined as the lowest sound pressure level to obtain wave V of the ABR visually. Judgments of visual detection were conducted by the same experienced physician who was unaware of any other clinical information.
Since the threshold of click-evoked ABR is well known to be correlated with the psychophysical threshold at 2,000 and 4,000 Hz [1920], the difference between the threshold of the ABR and the two-frequency average of the pure tone air-conduction thresholds at 2,000 and 4,000 Hz was used as an indicator of the degree of deterioration of the ABR.
Some patients had undergone DPOAE measurement with a GSI 70 Automated OAE System (RS 32 marketed by RION Company, Kokubunji, Tokyo, Japan) at 2f1-f2 carried out at 1,500, 2,000, 3,000, 4,000, 5,000, and 6,000 Hz of f2. The frequency ratio of f1/f2 was 1.2, and the levels of primaries were 65 dB (L1) and 55 dB (L2), respectively.
The relationship between the four-frequency PTA and the maximum speech intelligibility is shown in Fig. 1. Although a significant correlation was generally observable between the PTA and maximum speech intelligibility, the maximum speech intelligibilities appear to vary greatly, especially around the PTA level of 60 dB. To examine the relationships between speech intelligibilities among patients with similar levels of SNHL and threshold elevation of the ABR variety, the maximum speech intelligibility is plotted as a function of the deterioration of the ABR (threshold difference between the ABR and average pure tone thresholds at 2,000 and 4,000 Hz) in Fig. 2A, for 12 selected ears from 10 patients with PTA equal or greater than 55 dB and less than 65 dB (Fig. 1). There was a significant relationship between maximum speech intelligibility and deterioration of the ABR. Significant levels of DPOAEs could not be obtained in any of the patients in whom DPOAEs were examined (Fig. 2A, closed circles). Auditory thresholds (250-4,000 Hz) of these 12 ears are presented in Fig. 2B.
Although the data examined in the present retrospective study were relatively few and although further analysis based on a greater number of prospective data might be necessary, the present study found a significant correlation between deterioration of the ABR and maximum speech intelligibility consistent with our hypothesis that variation in speech intelligibilities among patients with similar levels of SNHL is related to the severity of the involved neural (cochlear nerve) and/or inner hair cell pathology in patients with SNHL.
In the present study, SNHL without apparent retrocochlear pathology was examined. However, even in these patients, every component in the auditory system, including the outer and inner hair cells (sensory), cochlear nerve (neural), stria vascularis (strial), etc., could be involved in the development of SNHL [21222324], although the outer hair cells are believed to be one of the most vulnerable component in most pathologies [2526272829]. Since dysfunctions of the inner hair cells and/or auditory neurons may be a cause of severe deterioration of ABR and speech intelligibility [34561213141516171821], the severity of inner hair cell and/or cochlear nerve damage is likely to be correlated with deterioration of speech intelligibility and/or the ABR in patients with SNHL.
Because deterioration of the ABR is observable from the early stage of various retrocochlear neural pathologies such as auditory neuropathy, vestibular schwannoma, etc. [345612131415161718], we assumed that the ABR could be a sensitive indicator to assess the presence or absence of retrocochlear type pathology (i.e., pathologies of inner hair cells and/or auditory nerves). Our present findings as shown in Fig. 2A appear to support this hypothesis that greater inner hair cell and/or cochlear nerve damage is associated with greater deterioration in speech intelligibility, even in patients with the same hearing threshold level.
Since the reduction of endocochlear potential affects both the inner and outer hair cells by reducing their driving voltage, the combined dysfunction of the inner and outer hair cells could also result from decreased endocochlear potentials because of dysfunction of the stria vascularis [2122]; i.e., disturbance of stria vascularis may be another factor causing greater deteriorated speech intelligibility as well as higher threshold of the ABR. Based on single unit studies, if the cochlear damage is limited to the outer hair cells, the response thresholds will not exceed around 60 dB and the neural response is even better than in the normal cochlea, and may respond to sound at the supra-threshold level [30]. On the other hand, neural response could deteriorate much more severely with involvement of inner hair cell damage and decreased endocochlear potentials [30], which presumably affect the formation of gross neural potentials (i.e., ABR). At present, the presence or absence of the involvement of stria vascularis is very difficult to distinguish clinically in patients with HL, but such disturbance of the stria vascularis may be present in patients with greater deterioration of the ABR and speech intelligibility among the patients with similar levels of HL.
ACKNOWLEDGMENTSThis study was supported by a grant from the Ministry of Education, Culture, Sports, Science and Technology-Japan (Grant-in-Aid for Exploratory Research 15659401).
CONFLICT OF INTERESTCONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported. References1. Fletcher H. A method of calculating hearing loss for speech from an audiogram. Acta Otolaryngol Suppl. 1950;90:26-37. PMID: 14818775.
2. Carhart R. Observations on relations between thresholds for pure tones and for speech. J Speech Hear Disord. 1971 11;36(4):476-483. PMID: 5125799.
3. Johnson EW. Auditory findings in 200 cases of acoustic neuromas. Arch Otolaryngol. 1968 12;88(6):598-604. PMID: 5724832.
4. Penrod JP. Speech threshold and word recognition/discrimination testing. In: Katz J, Gabbay WL, editors. Handbook of clinical audiology. 4th ed. Baltimore: Williams & Wilkins; 1994. p. 147-164.
5. Starr A, Picton TW, Sininger Y, Hood LJ, Berlin CI. Auditory neuropathy. Brain. 1996 6;119(Pt 3):741-753. PMID: 8673487.
6. Kaga K, Nakamura M, Shinogami M, Tsuzuku T, Yamada K, Shindo M. Auditory nerve disease of both ears revealed by auditory brainstem responses, electrocochleography and otoacoustic emissions. Scand Audiol. 1996;25(4):233-238. PMID: 8975994.
7. Amatuzzi M, Liberman MC, Northrop C. Selective inner hair cell loss in prematurity: a temporal bone study of infants from a neonatal intensive care unit. J Assoc Res Otolaryngol. 2011 10;12(5):595-604. PMID: 21674215.
8. Manchaiah VK, Zhao F, Danesh AA, Duprey R. The genetic basis of auditory neuropathy spectrum disorder (ANSD). Int J Pediatr Otorhinolaryngol. 2011 2;75(2):151-158. PMID: 21176974.
9. Santarelli R, Del Castillo I, Rodríguez-Ballesteros M, Scimemi P, Cama E, Arslan E, et al. Abnormal cochlear potentials from deaf patients with mutations in the otoferlin gene. J Assoc Res Otolaryngol. 2009 12;10(4):545-556. PMID: 19636622.
10. Liberman MC, Tartaglini E, Fleming JC, Neufeld EJ. Deletion of SLC19A2, the high affinity thiamine transporter, causes selective inner hair cell loss and an auditory neuropathy phenotype. J Assoc Res Otolaryngol. 2006 9;7(3):211-217. PMID: 16642288.
11. Ngo RY, Tan HK, Balakrishnan A, Lim SB, Lazaroo DT. Auditory neuropathy/auditory dys-synchrony detected by universal newborn hearing screening. Int J Pediatr Otorhinolaryngol. 2006 7;70(7):1299-1306. PMID: 16417926.
12. Kaga K, Iwasaki S, Tamura A, Suzuki J, Haebara H. Temporal bone pathology of acoustic neuroma correlating with presence of electrocochleography and absence of auditory brainstem response. J Laryngol Otol. 1997 10;111(10):967-972. PMID: 9425489.
13. Kveton JF. Delayed spontaneous return of hearing after acoustic tumor surgery: evidence for cochlear nerve conduction block. Laryngoscope. 1990 5;100(5):473-476. PMID: 2329903.
14. Michalewski HJ, Starr A, Nguyen TT, Kong YY, Zeng FG. Auditory temporal processes in normal-hearing individuals and in patients with auditory neuropathy. Clin Neurophysiol. 2005 3;116(3):669-680. PMID: 15721081.
15. Roberson JB Jr, Jackson LE, McAuley JR. Acoustic neuroma surgery: absent auditory brainstem response does not contraindicate attempted hearing preservation. Laryngoscope. 1999 6;109(6):904-910. PMID: 10369280.
16. Satya-Murti S, Wolpaw JR, Cacace AT, Schaffer CA. Late auditory evoked potentials can occur without brain stem potentials. Electroencephalogr Clin Neurophysiol. 1983 10;56(4):304-308. PMID: 6193943.
17. Starr A, McPherson D, Patterson J, Don M, Luxford W, Shannon R, et al. Absence of both auditory evoked potentials and auditory percepts dependent on timing cues. Brain. 1991 6;114(Pt 3):1157-1180. PMID: 2065245.
18. Takata Y, Kawase T, Nakasato N, Kanno A, Kobayashi T. Auditory evoked magnetic fields in patients with absent brainstem responses due to auditory neuropathy with optic atrophy. Clin Neurophysiol. 2012 5;123(5):985-992. PMID: 22119798.
19. Baldwin M, Watkin P. Predicting the degree of hearing loss using click auditory brainstem response in babies referred from newborn hearing screening. Ear Hear. 2013;May-Jun;34(3):361-369. PMID: 23340456.
20. van der Drift JF, Brocaar MP, van Zanten GA. The relation between the pure-tone audiogram and the click auditory brainstem response threshold in cochlear hearing loss. Audiology. 1987;26(1):1-10. PMID: 3593096.
21. Schuknecht HF, Gacek MR. Cochlear pathology in presbycusis. Ann Otol Rhinol Laryngol. 1993 1;102(1 Pt 2):1-16. PMID: 8420477.
22. Nadol JB Jr. Disorder of aging. In: Merchant SN, Nadol JB, editors. Schuknecht's pathology of the ear. 3rd ed. Shelton (CT): PMPH-USA; 2010. p. 431-476.
23. Adams JC, Merchant SN. Disorders of intoxication. In: Merchant SN, Nadol JB, editors. Schuknecht's pathology of the ear. 3rd ed. Shelton (CT): PMPH-USA; 2010. p. 353-380.
24. Merchant SN, Liberman MC. Trauma. In: Merchant SN, Nadol JB, editors. Schuknecht's pathology of the ear. 3rd ed. Shelton (CT): PMPH-USA; 2010. p. 381-412.
25. Kohonen A. Effect of some ototoxic drugs upon the pattern and innervation of cochlear sensory cells in the guinea pig. Acta Otolaryngol Suppl. 1965;(Suppl 208):1-70. PMID: 4159018.
26. Ylikoski J. Correlative studies on the cochlear pathology and hearing loss in guinea-pigs after intoxication with ototoxic antibiotics. Acta Otolaryngol Suppl. 1974;326:1-62. PMID: 4534029.
27. Schacht J, Talaska AE, Rybak LP. Cisplatin and aminoglycoside antibiotics: hearing loss and its prevention. Anat Rec (Hoboken). 012 11;295(11):1837-1850. PMID: 23045231.
28. Saunders JC, Dear SP, Schneider ME. The anatomical consequences of acoustic injury: a review and tutorial. J Acoust Soc Am. 1985 9;78(3):833-860. PMID: 4040933.
29. Merchant SN. Degeneration of auditory and vestibular end organs. In: Merchant SN, Nadol JB, editors. Schuknecht's pathology of the ear. 3rd ed. Shelton (CT): PMPH-USA; 2010. p. 631-664.
30. Kiang NY, Liberman MC, Sewell WF, Guinan JJ. Single unit clues to cochlear mechanisms. Hear Res. 1986;22(1-3):171-182. PMID: 3733538.
Fig. 1Relationship between the four-frequency average of the pure tone air-conduction thresholds at 500, 1,000, 2,000, and 4,000 Hz (four-frequency PTA) and the maximum speech intelligibility. Significant correlation was observed between the PTA and maximum speech intelligibility (coefficients of correlation assessed by IBM SPSS ver. 21.0 are shown in the lower right). Inserted vertical dotted lines at 55 and 65 dB indicate the region in which the maximum speech intelligibilities appear to be relatively varied and further analysis was conducted as shown in Fig. 2. PTA, pure tone average; HL, hearing loss.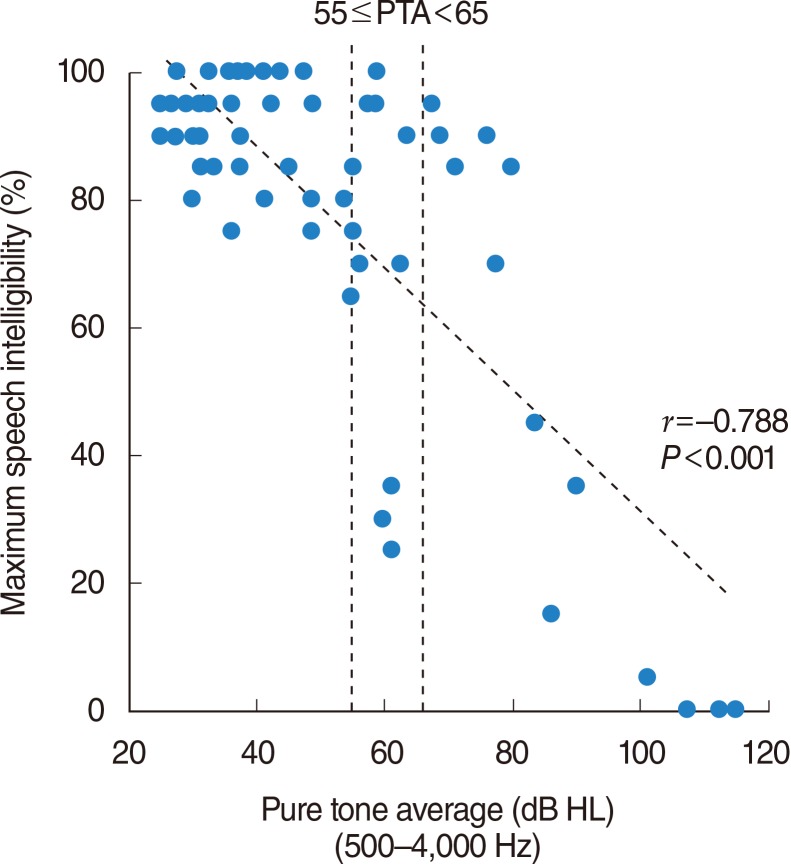
Fig. 2(A) Relationship between the maximum speech intelligibility and the degree of the deterioration of ABR (threshold difference between ABR and average pure tone thresholds at 2,000 and 4,000 Hz), in 12 selected ears from 10 patients with PTA equal or greater than 55 dB and less than 65 dB (Fig. 1). Significant correlation was observable (coefficients of correlation assessed by IBM SPSS ver. 21.0 are shown in the lower right). (B) Auditory thresholds (250-4,000 Hz) of these 12 ears are presented. Auditory thresholds of 4 ears with deteriorated ABR are shown as thick lines. ABR, auditory brainstem response; DPOAE, distortion product otoacoustic emission; PTA, pure tone average; HL, hearing loss.
|
|
|||||||||||||||||||||||||||||||||||||||||||







