|
 |
- Search
AbstractObjectivesInadequate antibody responses to pathogens may lead to the recurrence of otitis media with effusion (OME). Although B-cell production by antibodies is controlled by transcription factors, the status of these factors has not been assessed in patients with OME.
MethodsExpression of immunoglobulin was measured by enzyme-linked immunosorbent assay. Expression of transcription factors Bcl-6, Blimp-1, Pax-5, and XBP-1 was assessed by RT-PCR in the middle-ear fluid of 29 children with >4 OME episodes in 12 months or >3 episodes in 6 months (the OME-prone group) and in 32 children with <3 OME episodes in 12 months (OME group). The relationship between recurrence of OME and expression levels of immunoglobulins and transcription factors in middle-ear fluid was determined.
ResultsThe concentration of IgA in middle-ear fluid was significantly lower in the OME-prone than in the OME group, as was the expression of mRNA encoding the transcription factors Blimp-1 and XBP-1 (P<0.05 each). Expression of mRNA encoding the transcription factors Bcl-6 and Pax-5 was more intense in the OME-prone than in the OME group, but these differences were not significant (P>0.05).
Otitis media with effusion (OME) is characterized by the accumulation of fluid within the middle-ear cavity without acute symptoms such as fever or otalgia. Its etiology includes adenoid vegetation, allergic rhinitis, chronic sinusitis, Eustachian tube dysfunction due to cleft palate, bacterial infection and immune system defects (1, 2).
In patients with Eustachian tube dysfunction producing a ventilation defect, the air in the middle ear cavity is inhaled via a mucosal layer, generating a negative pressure in the middle ear cavity. Patients with persistent negative pressure show medial retraction of the tympanic membrane, as well as increased capillary permeability, due to swelling and vascular dilatation in the middle ear mucosa. This leads to exudate accumulation in the middle-ear cavity. The exudate becomes condensed over time, acting as a medium for bacterial cell growth and infection.
The fluid secreted from the middle ear mucosa in patients with otitis media has greater concentrations of glycoproteins and IgA than does the serum. In addition, mucus secreted by middle-ear goblet cells and glandular tissue has local defense functions (3).
The activity of antibodies involved in the defense mechanism of the middle-ear cavity is regulated by transcription factors that regulate the synthesis of antibodies. Among these transcription factors are B-cell leukemia lymphoma-6 (Bcl-6), B-lymphocyte inducer of maturation program 1 (Blimp-1), Paired box gene 5 (Pax-5) and X-box binding protein 1 (XBP-1). Bcl-6 and Pax-5 suppress, while Blimp-1 and XBP-1 enhance, immunoglobulin secretion. There are correlations among these transcription factors, in that Bcl-6 inhibits the activity of Blimp-1, Blimp-1 inhibits the activity of Pax-5, and Pax-5 inhibits the activity of XBP-1. Therefore, as the concentrations of Bcl-6 and Pax-5 are increased, those of Blimp-1 and XBP-1 are decreased (4).
In patients with OME, the type and amount of antibody in the effusion has a crucial defensive role in local immunity. Although many studies have assessed bacterial flora and antibody characteristics of effusion fluid, no studies to date have assessed the expression of transcription factors associated with the synthesis of antibodies. We therefore determined the concentrations of immunoglobulin and transcription factor mRNAs in the middle-ear fluid of patients with chronic OME and evaluated the differences between OME and OME-prone children.
The subject cohort consisted of 61 pediatric patients who visited the pediatric ear, nose and throat clinic of Kyung Hee University between March 2006 and February 2008 and underwent tympanostomy tube surgery for chronic OME. Subjects included 29 children with recurrent OME (>4 episodes in 12 months or >3 in 6 months; OME-prone group) and 32 with fewer episodes of OME who did not recover spontaneously (OME group). We divided the OME patients into those positive and negative for bacteria in effusion fluid by the bacterial culture method. The OME-prone group consisted of 17 boys and 12 girls, aged 5.3┬▒2.4 yr (mean┬▒standard deviation [SD] range, 4 to 11 yr). The OME group consisted of 20 boys and 12 girls, aged 4.9┬▒2.3 yr (range, 1 to 9 yr).
OME was diagnosed by the presence of an amber-colored tympanic membrane on otoscopic examination or by the presence of B- or C-type tympanograms as shown by impedance audiometry. Surgery was performed on chronic OME patients who did not show improvement after 2 weeks of antibiotic treatment and in patients who, after a 2-3 month follow up, showed progressive retraction of the eardrum or hearing loss progression, as shown by an increase in pure tone threshold. Children with head or neck deformities, systemic diseases, or those suspected of having congenital or acquired immune deficiencies were excluded from this study.
Prior to surgery, the external auditory canal was washed with potadine solution and effusion fluid was collected aseptically into an Eppendorf tube, being careful to avoid bleeding. Each effusion fluid sample was diluted 10 fold and centrifuged, and each supernatant and pellet were stored separately at -70Ōäā. Prior to surgery, the purpose of sample collection was explained to the parents or guardians of each child, and written informed consent was obtained.
Each effusion fluid sample was collected using sterilized cotton swabs, added to Stuart transport medium, and used to inoculate blood agar medium and thioglycollate liquid medium. The cultures were incubated for 24 hr at 35Ōäā, and resultant bacteria were identified by gram staining and biochemical tests.
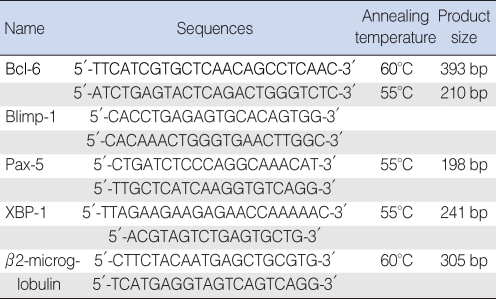
Gene expression was quantified using real-time polymerase chain reaction (PCR) (Roche Diagnostics, Mannheim, Germany). To establish relative measures for Bcl-6, Blimp-1, Pax-5 and XBP-1, we first used primers for ╬▓-2 microglobulin based on sequences in GenBank. Each primer pair spanned an intron to remove the effects of any genomic DNA contaminants (Table 1). The number of threshold cycles for each target gene was obtained, and the crossing point of each with ╬▓-2 microglobulin was entered into the formula 2-(target gene-╬▓2 microglobulin) for relative quantification.
All middle-ear effusion samples were stored at -80Ōäā, and the concentrations of IgG, IgA, and IgM in each sample were measured by ELISA. Briefly, 50 ┬ĄL of 1:100 goat anti-human IgG, anti-human IgA, or anti-human IgM in coating buffer (1.59 g Na2CO3, 2.93 g NaHCO3, 5% NaN3, pH 9.6) were placed in each well of a 96-well plate and incubated overnight at 4Ōäā. The wells were washed six times, blocking antibody was added and 50 ┬ĄL of sample was added to each well followed by incubation at room temperature for 3 hr. The wells were washed six times, purified goat anti-human IgG, IgA, or IgM conjugated to horseradish peroxidase in PBS/Tween/BSA solution was added, and the plates were incubated at room temperature. The plates were washed six times, substrate solution (2,2'-AZINO-Bis) was added, and the optical absorbance was measured at 450 nm (Bethyl, Montgomery, TX, USA).
The mean relative levels of Blimp-1 (22.7┬▒1.8 vs. 24.3┬▒1.8, P<0.05) and XBP-1 (22.9┬▒1.9 vs. 24.2┬▒1.7, P<0.05) mRNA were significantly lower in the OME-prone than in the OME group. In contrast, the mean relative levels of Bcl-6 (25.9┬▒2.5 vs. 25.6┬▒2.1) and Pax-5 (23.7┬▒2.3 vs. 23.3┬▒1.9) mRNA were slightly higher in the OME-prone than in the OME group, but these differences were not statistically significant (P>0.05) (Fig. 1, Table 2).
Bacteria were detected in 15/61 (24.5%) effusion fluid samples. Among the bacteria detected by bacterial culture were S. pneumonia (4 patients), H. influenzae (3 patients), M. catarrhalis (3 patients), MRSA (3 patients), coagulase negative Staphylococcus (2 patients), and P. aeruginosa (1 patient).
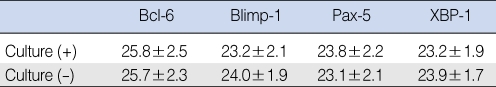
The mean relative levels of Blimp-1 (23.2┬▒2.1 vs. 24.0┬▒1.9) and XBP-1 (23.2┬▒1.9 vs. 23.9┬▒1.7) mRNA were lower in the culture (+) than in the culture (-) group. The mean relative levels of Bcl-6 (25.8┬▒2.5 vs. 25.7┬▒2.3) and Pax-5 (23.8┬▒2.2 vs. 23.1┬▒2.1) mRNA were slightly higher in the culture (+) than in the culture (-) group, but none of these differences was statistically significant (P>0.05) (Table 3).
When we measured the overall concentration of immunoglobulins in the ear fluid of patients with chronic OME, we found that the concentration of IgA was significantly lower in the OME-prone than in the OME group (330┬▒248 ng/mL vs. 668┬▒425 ng/mL, P<0.05) (Table 4). In contrast, although the concentrations of IgG (1,775┬▒1,469 ng/mL vs. 2,983┬▒2,371 ng/mL) and IgM (677┬▒680 ng/mL vs. 1,568┬▒762 ng/mL) were lower in the OME-prone than in the OME group, these differences were not statistically significant.
Clinically, otitis media has been found to recur frequently in some pediatric patients. The otitis-prone condition, defined as >3 episodes in 6 months or >4 in one year, has been observed in approximately 5% of pediatric patients with OME (5). Chronic symptoms of OME are due mainly to secondary changes and the resulting dysfunction of the Eustachian tubes (1, 2). These secondary changes include the impaired production of antibodies, cilia dysfunction, mucosal swelling and hyperplasia, mucoid changes in secretions, fibrosis, edema and overproduction of mucus due to increased numbers of goblet cells and glandular tissue. Recent studies in patients with OME have assessed bacteria, cells, cytokines and antibodies in the middle-ear fluid (3, 6-8). Chronic OME frequently occurs after recurrent acute otitis media (AOM) or upper respiratory infection. Therefore bacterial infection is suggested as the major cause of chronic OME (9). Sloyer et al. (10) reported that a negative bacterial culture is correlated with the level of specific antibody, indicative of the eradication of bacteria by specific antibody. We attempted to evaluate the level of transcription factor for target antibodies, not the levels of specific antibodies, since we did not perform bacterial DNA fingerprinting. Therefore we could not determine whether the chronic OME is a recurrent infection or another infection (11).
Although extraction of middle-ear mucosa would provide samples for accurate, detailed evaluation of the status of the middle-ear cavity, middle-ear mucosa cannot be extracted for ethical reasons. Thus, although our methods were indirect, evaluating middle-ear fluid from patients, it provides accurate concentrations of transcription factors and immunoglobulins in the middle-ear fluid of patients with OME. Studies performed in patients with acute otitis media have shown that IgA concentrations are higher in middle-ear fluid than in blood of patients with OME, whereas the concentrations of IgM and IgG are lower in middle-ear fluid than in serum (8, 10).
The concentrations of IgG2 and IgA have been reported to be lower in the serum and middle ear fluid of patients with recurrent OME than in those with OME (12). Moreover, the serum and middle ear fluid concentrations of IgG specific for Moraxella catarrhalis were found to be lower in OME-prone than in OME patients (13). In those reports, however, the causes of OM, infected organs, and types of antibody were diverse. In our study we evaluated the correlation between IgA concentration and transcription factors for antibodies in chronic OME patients who did not respond to drug treatment for 2 weeks and with a 3 month follow-up.
Unlike IgG or IgM, IgA is primarily synthesized by plasma cells present in the respiratory and intestinal mucosae and in glandular tissue. Thus, although some IgA is present in the blood, most is present in the epithelium or the spaces between epithelia as secretory immunoglobulin A (sIgA). In patients with acute otitis media, bacterial stimulation of local immunity in the middle-ear cavity leads to the increased synthesis of sIgA. However, in patients with decreased concentrations of IgA due to defects in its production, the impairment of local immunity in the middle-ear cavity causes the recurrence and chronic changes observed in patients with OME. In support of this finding, we also observed that the IgA concentration was significantly lower in the OME-prone than in the OME group.
Inflammatory cells observed in the middle-ear fluid of patients with OME, whether of serous or mucous origin, consist of 50% neutrophils, 30% macrophages and 20% lymphocytes (5, 6). Neutrophils and macrophages, which are components of the innate immune system, directly engulf the pathogens. The innate immune system shows a shorter time to response and a wider range of antibacterial activity than the acquired immune system, which involves antibodies and immune system cells. B-lymphocytes, which are responsible for humoral immunity in the acquired immune system, respond to specific antigens and produce antibodies. These antibodies neutralize the antigens in vivo and then remove them, according to pathways specific for each antibody isotype (14). In the last stage of antigenic stimulation, B-lymphocytes differentiate into plasma cells in the germinal center. Plasma cells produce high-affinity antibodies and often survive in the bone marrow for several months. The transformation of B-2 cells into plasma cells involves four transcription factors involved in the production of immunoglobulins, Bcl-6, Blimp-1, Pax-5 and XBP-1 (4, 15-19). Bcl-6 and Pax-5 suppress the differentiation of B-cells into plasma cells in the germinal center, as well as suppressing the production of antibodies by plasma cells (15, 17). In contrast, Blimp-1 and XBP-1 are transcription factors that terminate the cell cycle and promote B-cell differentiation.
In addition to inhibiting B-cell differentiation, Bcl-6 inhibits the expression of genes involved in B-cell activity and those that encode the cyclin-dependent kinase (cdk) inhibitors, p27 and p21. Thus, Bcl-6 suppresses prompt cell differentiation. The major role of Bcl-6 is to suppress the expression of Blimp-1 and the differentiation of B-cells into plasma cells.
Blimp-1 induces the expression of the cdk inhibitor p18, which is needed for differentiation into plasma cells, the proapoptotic genes GADD45 and GADD153, and J chain, XBP-1 and HSP-70 mRNAs, all of which are involved in the secretion of immunoglobulins (16). We found that Blimp-1 mRNA expression and the concentration of IgA were significantly lower in the OME-prone than in the OME group. Blimp-1 may suppress antibody production, causing decreased immunity to bacterial and viral antigens, thus providing clues to its involvement in the progression of chronic OME.
Like Bcl-6, Pax-5 is needed to suppress B-cell differentiation into plasma cells in the germinal center and to suppress antibody production. Pax-5 can activate or inhibit the activity of transcription factors. However, we found no intergroup differences in the concentrations of Pax-5 and Bcl-6. This may be due to persistent, repetitive antigen stimulation, which may lead to the expression of transcription factors involved in the suppression of immunoglobulin secretion. Reduced expression of Pax-5 may induce expression of XBP-1 and immunoglobulin heavy chain, suggesting that, in B-lymphocytes, the overproduction of Pax-5 suppresses the differentiation of plasma cells and decreases antibody production (17).
In the absence of XBP-1, T-cells and B-cells develop normally and the formation of germinal centers and the production of cytokines are normal. However, lymphocyte production of immunoglobulins is severely impaired, suggesting that XBP-1 is an essential factor for differentiation into plasma cells. We found that the expression of XBP-1 mRNA was significantly lower in the OME-prone than in the OME group, suggesting that decreased production of antibodies is related to the recurrence of OME.
The levels of Blimp-1 and XBP-1 were lower in the culture (+) group than in the culture (-) group but these differences were not statistically significant. Increased levels of specific antibodies for S. pneumoniae and H. influenza in middle ear fluid have been reported to correlate with negative bacterial cultures in acute OM patients, indicating that the bacteria had been eliminated by a specific antibody (10). Although not statistically significant, the lower levels of Blimp-1 and XBP-1 in the culture (+) group strongly suggest that the decreased level of antibody specific transcription factor was associated with decreased antibody production, thus reducing the rates of elimination of bacteria and specific antibody. In general, PCR is used to identify bacteria, showing a greater than 2-fold sensitivity compared with conventional culture methods (20). PCR, however, can also detect the presence of a previous infection or inactive bacteria. The addition of PCR may result in the more accurate evaluation of antibody transcription factor.
This study is the first to assess this previously established paradigm in the fluid of patients with recurrent OME. We found that the expression of Blimp-1 and XBP-1 mRNA was significantly lower in the OME-prone than in the OME group. Moreover, despite a lack of statistical significance, the expression levels of Bcl-6 and Pax-5 mRNA were higher in the OME-prone than in the OME group. These results are in agreement with the previous paradigm for transcription factors (4, 15-19). They suggest that this paradigm is applicable to infections the middle-ear cavity. Our findings also suggest that expression of Bcl-6 and Pax-5 suppresses antibody production, whereas expression of Blimp-1 and XBP-1 promotes it. Impaired production of antibodies against antigens invading the middle-ear cavity may be related to the recurrence and chronic changes observed in patients with recurrent OME.
We have observed expression of mRNAs encoding transcription factors associated with B-cell antibody production in the middle-ear fluid of pediatric patients with chronic OME. Decreased levels expression of Blimp-1 and XBP-1 mRNA are associated with impaired production of IgA in OME prone patients.
NotesThis Research was Supported by the Program of Kyung Hee University for the Young Researcher in Medical Science (KHU-1249). References1. Cummings CW, Flint PW, Harker LA, Haughey BH, Richardson MA, Robbins KT, et al. . Cummings otolaryngology: head and neck surgery. 2005. 4th ed. Philadelphia: Mosby Co.
2. Bluestone CD, Klein JO. Clinical practice guideline on otitis media with effusion in young children: strengths and weaknesses. Otolaryngol Head Neck Surg. 1995 4;112(4):507-511. PMID: 7700654.
3. Howie VM, Ploussard JH, Sloyer JL, Johnston RB Jr. Immunoglobulins of the middle ear fluid in acute otitis media: relationship to serum immunoglobulin concentrations and bacterial cultures. Infect Immun. 1973 4;7(4):589-593. PMID: 4148623.
4. Tumang JR, Frances R, Yeo SG, Rothstein TL. Spontaneously Ig-secreting B-1 cells violate the accepted paradigm for expression of differentiation-associated transcription factors. J Immunol. 2005 3;174(6):3173-3177. PMID: 15749846.
5. Howie VM, Ploussard JH, Sloyer J. The otitis-prone condition. Am J Dis Child. 1975 6;129(6):676-678. PMID: 239591.
6. Skotnicka B, Hassmann E. Cytokines in children with otitis media with effusion. Eur Arch Otorhinolaryngol. 2000;257(6):323-326. PMID: 10993552.
7. Juhn SK, Sipil├ż P, Jung TT, Edlin J. Biochemical pathology of otitis media with effusion. Acta Otolaryngol Suppl. 1984;414:45-51. PMID: 6598270.
8. Yamanaka N, Somekawa Y, Suzuki T, Kataura A. Immunologic and cytologic studies in otitis media with effusion. Acta Otolaryngol. 1987;NovŌĆōDec;104(5-6):481-486. PMID: 3434270.
9. Liu YS, Lang R, Lim DJ, Birck HG. Microorganism in chronic otitis media with effusion. Ann Otol Rhinol Laryngol. 1976;85(2):Suppl 25. 245-249. PMID: 5041.
10. Sloyer JL Jr, Howie VM, Ploussard JH, Schiffman G, Johnston RB Jr. Immune response to acute otitis media: association between middle ear fluid antibody and the clearing of clinical infection. J Clin Microbiol. 1976 9;4(3):306-308. PMID: 9424.
11. Ruohola A, Meurman O, Nikkari S, Skottman T, Salmi A, Waris M, et al. Microbiology of acute otitis media in children with tympanostomy tubes: prevalences of bacteria and viruses. Clin Infect Dis. 2006 12;43(11):1417-1422. PMID: 17083014.
12. Freijd A, Hammarstr├Čm L, Persson MA, Smith CI. Plasma anti-pneumococcal antibody activity of the IgG class and subclasses in otitis prone children. Clin Exp Immunol. 1984 5;56(2):233-238. PMID: 6733969.
13. Takada R, Harabuchi Y, Himi T, Kataura A. Antibodies specific to outer membrane antigens of Moraxella catarrhalis in sera and middle ear effusions from children with otitis media with effusion. Int J Pediatr Otorhinolaryngol. 1998 12;46(3):185-195. PMID: 10190589.
14. Ichimiya I, Kawauchi H, Mogi G. Analysis of immunocompetent cells in the middle ear mucosa. Arch Otolaryngol Head Neck Surg. 1990 3;116(3):324-330. PMID: 2407271.
15. Kusam S, Dent A. Common mechanisms for the regulation of B cell differentiation and transformation by the transcriptional repressor protein BCL-6. Immunol Res. 2007;37(3):177-186. PMID: 17873402.
16. Angelin-Duclos C, Cattoretti G, Lin KI, Calame K. Commitment of B lymphocytes to a plasma cell fate is associated with Blimp-1 expression in vivo. J Immunol. 2000 11;165(10):5462-5471. PMID: 11067898.
17. Lin KI, Angelin-Duclos C, Kuo TC, Calame K. Blimp-1-dependent repression of Pax-5 is required for differentiation of B cells to immunoglobulin M-secreting plasma cells. Mol Cell Biol. 2002 7;22(13):4771-4780. PMID: 12052884.
18. Shaffer AL, Yu X, He Y, Boldrick J, Chan EP, Staudt LM. BCL-6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity. 2000 8;13(2):199-212. PMID: 10981963.
19. Ozaki K, Spolski R, Ettinger R, Kim HP, Wang G, Qi CF, et al. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol. 2004 11 01; 173(9):5361-5371. PMID: 15494482.
20. Yeo SG, Park DC, Lee SK, Cha CI. Relationship between effusion bacteria and concentrations of immunoglobulin in serum and effusion fluid in otitis media with effusion patients. Int J Pediatr Otorhinolaryngol. 2008 3;72(3):337-342. PMID: 18242717.
Fig.┬Ā1Expression of Bcl-6, Pax-5, XBP-1, and Blimp-1 mRNAs in middle-ear cavity fluid of patients in the otitis media with effusion (OME) and OME-prone groups. Polymerase chain reaction products were separated on a 2% agarose gel and stained with ethidium bromide. N: normal peripheral blood, P: middle-ear cavity fluid. 
Table┬Ā2Relative transcription factor level in middle-ear cavity fluid in the otitis media with effusion (OME) and OME-prone groups 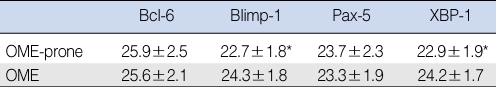
|
|
||||||||||||||||||||||||||||||||||||