 |
 |
- Search
AbstractObjectives To investigate the expression of prostaglandin E2 receptor subtypes, E-prostanoid (EP) 1ŌĆō4 receptors, in acquired cholesteatoma and its possible role in the pathologic process of this disorder.
Methods Specimens of human acquired cholesteatoma were obtained from 29 patients and 19 skin biopsies of normal external auditory canal were as controls. The mRNA and protein expression of EP receptors was assessed by quantitative real-time polymerase chain reaction, immunohistochemistry and Western blot.
Results In acquired cholesteatoma, EP1ŌĆōEP4 receptors were mainly expressed on squamous epithelium and subepithelial infiltrated inflammatory cells. In external auditory canal skin, EP1ŌĆōEP4 receptors were mainly expressed on squamous epithelium and glandular epithelium. The expression of EP4 receptor on mRNA and protein levels were significant lower in acquired cholesteatoma compared with controls. EP1ŌĆōEP3 receptors had no significant difference between the experimental and control group.
Middle ear cholesteatoma is a mass of keratin-producing squamous epithelium in the middle ear, which has been divided into congenital cholesteatoma and acquired cholesteatoma according to their theoretical mechanism of pathogenesis [1]. Acquired cholesteatoma has a history of primary or secondary otitis media. It is a chronic inflammatory disease usually accompanied with tympanic membrane perforation or inverted cysts and characterized by bone destruction.
The etiology of cholesteatoma is complex and multifactorial. Previous studies suggest the hyperproliferation and bone destruction of cholesteatoma are directly or indirectly related to inflammatory mediators. Cytokines, chemokines, prostaglandins, and leukotrienes which were generated by lymphocytes, monocyte, neutrophils, and keratinocytes in cholesteatoma may cause tissue damage in the chronic inflammatory process [2]. For example, activation of interleukin (IL)-6/JAK/STAT3 signaling pathway played a crucial role in the epithelial hyperplasia of cholesteatoma [3]. Some studies also suggested IL-1╬▒, tumor necrosis factor-╬▒ (TNF-╬▒), and prostaglandins are released to stimulate osteoclasts formation and bone resorption in cholesteatoma [4]. However, the exact pathological mechanism of middle ear cholesteatoma is still unclear.
Prostaglandin E2 (PGE2) is one of the most important members of prostaglandin family. It is a well-known inflammatory and bone metabolism mediator, which plays various roles in different types of inflammatory and bone-related disorders through four E-prostanoid (EP) receptors, EP1, EP2, EP3, and EP4. Each EP receptor mediates a specific intracellular signaling pathway to perform different functions. In the acquired cholesteatoma, several researchers found that the concentration of PGE2 in cholesteatoma is significant higher than granulation tissue [5] and PGE2 may promote bone erosion in cholesteatoma [6]. However, EP receptor expression of cholesteatoma has not been reported and it is not yet clear which receptors were involved in the pathological process of cholesteatoma. Therefore, the present study was designed to investigate the expression profiles of EP receptors in acquired cholesteatoma and understand the possible role of EP receptors in the pathological process of this disorder.
This study was approved by the ethics committee of Tongji Medical College of Huazhong University of Science and Technology, Wuhan, China (approval No. TJ-C20130312), and was conducted with written informed consent from patients. The cholesteatoma samples from 29 patients (mean age, 28.9 years; range, 3 to 64 years; female, 43.5%) with acquired cholesteatoma were as experimental group and 19 external auditory canal skin specimens (mean age, 25.5 years; range, 3 to 49 years; female, 42.1%) as control group. The diagnoses of acquired cholesteatoma in the experimental group were confirmed by pathologic examination and clinical data. Specimens were collected during the cholesteatoma surgery on a dry ear. Control group were also from the acquired middle ear cholesteatoma patients without inflammation in external auditory canal.
Specimens were fixed in 4% paraformaldehyde, embedded in paraffin and cut into 4 ╬╝m. Hematoxylin-eosin stain was applied to confirm the specimens contained the squamous epithelium and subepithelium. After deparaffinization and rehydration, the tissue sections were treated in a microwave oven for antigen retrieval. Endogenous peroxidase activity was blocked by 3% hydrogen peroxidase. Then, the tissue slides were treated with 5% bovine serum albumin to block nonspecific binding sites. Sections were incubated with polyclonal rabbit antihuman EP receptors (EP1ŌĆōEP4 at a dilution of 1:100; Cayman Chemical, Ann Arbor, MI, USA) overnight at 4┬░C. A secondary antibody and a streptavidin-biotin horseradish peroxidase kit (GK600510, Gene Tech, Shanghai, China) were used for immunohistochemical staining. The sections were washed with phosphate buffered saline. The immunoreactions were visualized by using 3,3-diaminobenzidine tetrahydrochloride, which stained positive cells brown. Negative control slides had the same immunohistologic treatment with substitution of the primary antibody with no immune sera of the same species.
Total RNA was obtained from specimens with a RNA extraction kit according to product instructions (Tiangen Biotech, Beijing, China). cDNA was prepared using PrimeScript RT reagent kit with gDNA eraser (Takara Bio Inc., Otsu, Japan). Then quantitative real-time polymerase chain reaction (PCR) was run on LightCycler 480 II (Roche, Basel, Switzerland) using SYBR Premix Ex Taq (Takara Bio Inc.). Primers used for quantitative real-time PCR were show in Table 1. The quantitative real-time PCR reaction condition for EP1ŌĆōEP4 was 40 cycles, denaturation at 95┬░C for 10 seconds, annealing at 60┬░C for 10 seconds, and extension at 72┬░C for 15 seconds. The expression of the four receptors was normalized to housekeeping gene GAPGH for each sample. Every reaction has three duplicates. Ct value is small enough and similar between the duplicates. Melting curve was analyzed to rule out contamination.
The cholesteatoma and external auditory canal skin tissues were lysed with lysis buffer (RIPA and protease inhibitors) in homogenizers to get total proteins. Quantified protein (40 ╬╝g) was mixed with loading buffer, boiled for 5 minutes to denature, subjected to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred to polyvinylidene difluoride membranes. After blocking with 5% nonfat milk in Tris-buffered saline for 2 hours at room temperature, the membranes were incubated with polyclonal rabbit antihuman EP receptors (EP1 at 1:1,000, EP2 at 1:1,200, EP3 at 1:1,500, and EP4 at 1:500; Cayman Chemical) overnight at 4┬░C. Then the membranes were washed with Tris-buffered saline and incubated with horseradish peroxidase-linked secondary antibodies (1:3,000 dilution; Goodbio, Wuhan, China). The signals were examined by using a chemiluminescent method (Pierce Chemical, Rockford, IL, USA). Protein levels were semiquantified. The target protein was normalized to glyceraldehyde phosphate dehydrogenase. GeneTools 4.0 software (Syngene, Cambridge, UK) was used to quantify protein band intensities.
Data were analyzed by Graphpad Prism Software ver. 5.0 (Graphpad Software, San Diego, CA, USA) and SPSS ver. 19.0 (IBM Corp., Armonk, NY, USA). The significant expression differences of the EP1, EP2, EP3, and EP4 receptors between the cholesteatoma and the skin were analyzed with the Mann-Whitney U-test. Statistical significance was concluded at P-value<0.05.
The immunohistochemistry staining showed positive reaction of EP1ŌĆōEP4 receptors in acquired cholesteatoma and skin. EP1ŌĆōEP4 receptors showed a similar cellular localization in both groups. The four receptors were all positive on squamous epithelium of skin and cholesteatoma. Besides, EP2 and EP3 receptors were also expressed on the blood vessels in acquired cholesteatoma and control subject. There were some differences in EP expression between two groups. In acquired cholesteatoma, EP1ŌĆōEP4 receptors were expressed on the infiltrated inflammatory cells. In skin, positive reaction of the four receptors were also found on subepithelial gland (Fig. 1).
We tested the EP receptor subtypes mRNA expression in both groups with quantitative real-time PCR. In comparison with control group, the mRNA expression level of EP4 receptor was significantly lower in cholesteatoma (P=0.016) (Fig. 2). There were no significant differences in mRNA expression level of EP1, EP2, and EP3 receptors between the two groups.
Western blot analysis revealed that EP1ŌĆōEP4 were present in cholesteatoma and the external auditory canal skin at detectable levels. The level of EP4 receptors were significantly downregulated in acquired cholesteatoma specimens compared with controls (P=0.002) (Fig. 3). There was no significant difference in the protein levels of EP1ŌĆōEP3 receptors between the cholesteatoma and the skin.
In this study, we first demonstrate the expression profiles of the four EP receptor subtypes in human acquired cholesteatoma and skin of the external acoustic meatus. We found EP1ŌĆōEP4 were positive on structural cells (epithelium, glands, and/or blood vessels) in external auricular canal skin. As for cholesteatoma, our findings showed that EP receptors were expressed not only on structural cells (epithelium and/or blood vessels) but also on subepithelium inflammatory cells. The different localization of EP1ŌĆōEP4 receptors between cholesteatoma and skin may be explained by the differences in histopathology because of the lack of glandular structure in cholesteatoma. More importantly, amounts of infiltrating inflammatory cells play a crucial role in the inflammatory reaction of cholesteatoma as described [2]. The differences in cellular distributions of EP receptors maybe link with aggressive and destructive pathological processes in cholesteatoma.
Our results showed that the mRNA and protein expression of EP4 receptor was significantly decreased in cholesteatoma in comparison with the skin. EP4 receptor is involved in various physiological and pathological processes. For example, EP4 receptor mediated vascular relaxation [7] and the closure of ductus arteriosus [8]. Most studies demonstrated that EP4 mainly played a crucial anti-inflammatory role in inflammatory diseases [9-11]. PGE2 suppressed expression of various inflammatory chemokines including macrophage inflammatory protein-1╬▒ and -1╬▓, monocyte chemoattractant protein-1, IL-8, TNF-╬▒, and interferon-╬│ in human macrophages by EP4 receptor in atherosclerosis [9]. EP4-selective antagonist induced obvious proliferation of CD4+ T cells and inhibited the recovery of colonitis [10]. Structural cells (epithelium and vascular endothelium), local cells such as keratinocytes and mast cells as well as infiltrating inflammatory cells in cholesteatoma are the sources of a large number of proinflammatory factors including chemokines, cytokines, and prostaglandins [2]. Excess proinflammatory mediators cause inflammatory lesions and contribute to destruction of the bone and ossicles. Based on this knowledge, we consider that the reduced expression of EP4 receptors may aggravate the inflammation response of cholesteatoma due to its decreased anti-inflammatory effect.
One of the most distinct characteristics of cholesteatoma is bone destruction, which may cause auditory ossicles and surrounding bone resorption, leading to the serious consequences of hearing loss, vestibular dysfunction, facial paralysis, intracranial complications, and so on. Bone metabolism is up to the balance of osteoblasts and osteoclasts. PGE2 is one of the most important mediators involved in bone metabolism; several studies suggested PGE2 promoted bone formation in specific dosage in vivo [12-14] and in vitro [15,16]. By adopting selective agonists and antagonists of EP4 receptor, researchers have found that PGE2 elevated the number of bone marrow osteogenic stromal cells, involved in differentiation and recruitment of osteoblasts, and exerted anti-apoptosis effect on periosteal cell line [17-20]. Moreover, EP4 receptor plays a crucial role in anabolic action on bone [12,13,16]. However, other studies indicated PGE2 could increase the number of osteoclasts [21] and EP4 receptor was expressed on osteoblasts but also was involved in osteoclasts differentiation [4]. Therefore, the exact role of PGE2 on bone metabolism seems to be unclear. It is worth noting that EP4 was the only functional receptors found in human osteoblasts in culture of the four receptors [22], which activated osteoblasts directly and osteoclasts indirectly [23]. Activation and increasing number of osteoclasts might associate with the new bone formed by osteoblasts [24]. The down expression of EP4 receptor in cholesteatoma suggested that bone anabolic action could be attenuated and the bone resorption process might correspondingly be exacerbated. On the other hand, decreased expression of EP4 receptor may aggravate the inflammation response, leading to the increase of inflammatory cells and chemokines such as CD4+ T cells [10] and TNF-╬▒ [9]. And cholesteatoma debris also promoted the expression of TNF-╬▒ in monocytes [25]. TNF-╬▒ is a widely accepted cytokine generated by monocytes and lymphocytes, which caused osteoclastic bone resorption. Therefore, we consider EP4 receptor played an important role in the bone destruction of acquired cholesteatoma. It could be expected that EP4 receptor may become a possible therapeutic target for bone destruction of this disorder, which needs to be explored by future studies.
In the present study, the expression of EP1, EP2, or EP3 receptors did not show significant differences between two groups. Several studies reported that EP1, EP2, and EP3 receptors were also involved in some inflammatory diseases. EP1 was highly expressed in eosinophilic chronic rhinosinusitis with nasal polyps [26]. EP3 receptor elicited histamine release to promote inflammatory swelling through mast cell [27] and mediated adhesion of mast cell to the Arg-Gly-Asp-enriched matrix [28]. EP1 and EP3 mainly played a proinflammatory effect in these studies. It also found that EP2 receptor agonists presented an anabolic action on bone (primarily through P38 mitogen-activated protein kinases pathway) and its intracellular transduction pathway was different from EP4 receptor (mainly by extracellular signal-regulated kinases signaling) [29]. The differential expression of the four receptors might result from the specific activation of the involved cell types and intracellular transduction pathway in different lesions. No difference in the expression of EP1ŌĆōEP3 receptors in cholesteatoma and skin suggests that these receptors may not be associated with inflammatory and destructive pathologic process of this disorder. The pathophysiology function of PGE2 in acquired cholesteatoma might mediate mainly through EP4 receptor. More detailed intracellular signaling pathway needs to be further investigated.
In the present study, we demonstrated the cellular distribution of EP1ŌĆōEP4 and their expression on the mRNA and protein level in acquired cholesteatoma. Reduced expression of EP4 receptor might play a crucial role in the inflammation and bone resorption processes of acquired cholesteatoma.
Fig.┬Ā1.Immunohistochemical localization of the (A-D) EP1ŌĆōEP4 receptors and (E) negative control (NC) in acquired cholesteatoma (CHOL) and external auditory canal skin (SKIN) (├Ś400). EP2 and EP3 receptors were positive in vascular endothelium (bold arrows). EP1ŌĆōEP4 receptors were also expressed on and residues of the middle ear mucosa (thin arrows, data EP1 and EP2 not shown). Red arrows showed the inflammatory cells. Brown staining represents positive expression. EP, E-prostanoid. 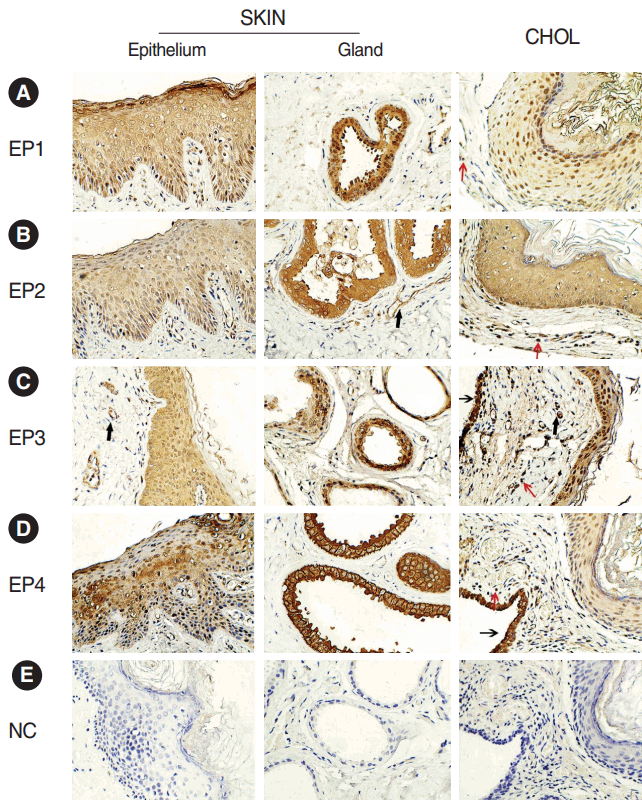
Fig.┬Ā2.Comparison of mRNA expression of EP1, EP2, EP3, and EP4 receptors between external auditory canal skin (SKIN) and cholesteatoma (CHOL). The expression of EP4 receptor, but not EP1ŌĆōEP3 receptors, was significantly reduced in cholesteatoma. EP, E-prostanoid. *P<0.05. 
Fig.┬Ā3.(A) Representative immunoblots of the four EP receptors and (B) comparison of protein expression of EP receptors between external auditory canal skin (SKIN) and cholesteatoma (CHOL). EP, E-prostanoid; GAPDH, glyceraldehyde phosphate dehydrogenase. *P<0.05. 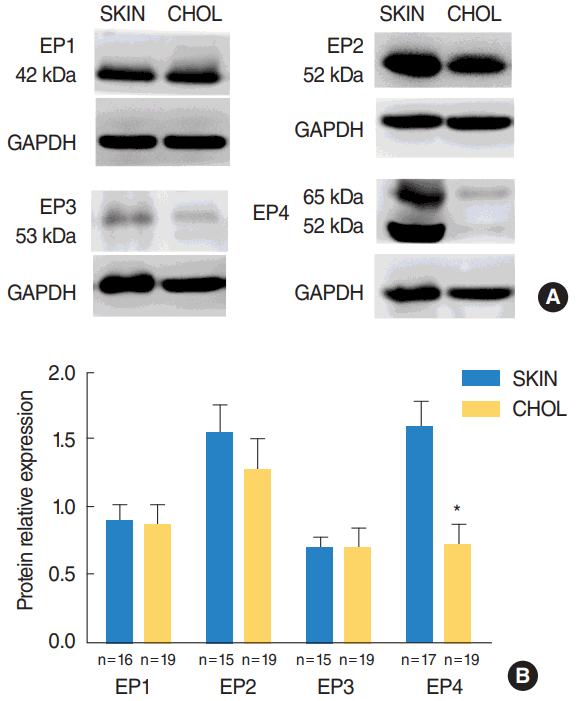
Table┬Ā1.Primer sequences used for real-time PCR amplifications REFERENCES1. Olszewska E, Wagner M, Bernal-Sprekelsen M, Ebmeyer J, Dazert S, Hildmann H, et al. Etiopathogenesis of cholesteatoma. Eur Arch Otorhinolaryngol. 2004 Jan;261(1):6-24.
2. Juhn SK, Jung MK, Hoffman MD, Drew BR, Preciado DA, Sausen NJ, et al. The role of inflammatory mediators in the pathogenesis of otitis media and sequelae. Clin Exp Otorhinolaryngol. 2008 Sep;1(3):117-38.
3. Liu W, Xie S, Chen X, Rao X, Ren H, Hu B, et al. Activation of the IL-6/JAK/STAT3 signaling pathway in human middle ear cholesteatoma epithelium. Int J Clin Exp Pathol. 2014 Jan;7(2):709-15.
4. Sakuma Y, Tanaka K, Suda M, Yasoda A, Natsui K, Tanaka I, et al. Crucial involvement of the EP4 subtype of prostaglandin E receptor in osteoclast formation by proinflammatory cytokines and lipopolysaccharide. J Bone Miner Res. 2000 Feb;15(2):218-27.
5. Jung TT, Juhn SK. Prostaglandins in human cholesteatoma and granulation tissue. Am J Otol. 1988 May;9(3):197-200.
6. Shirahata Y, Abramson M. Experimental aural cholesteatoma causing bone resorption. Nihon Jibiinkoka Gakkai Kaiho. 1981 Nov;84(11):1451-9.
7. Hristovska AM, Rasmussen LE, Hansen PB, Nielsen SS, Nusing RM, Narumiya S, et al. Prostaglandin E2 induces vascular relaxation by E-prostanoid 4 receptor-mediated activation of endothelial nitric oxide synthase. Hypertension. 2007 Sep;50(3):525-30.
8. Yokoyama U, Minamisawa S, Quan H, Ghatak S, Akaike T, Segi-Nishida E, et al. Chronic activation of the prostaglandin receptor EP4 promotes hyaluronan-mediated neointimal formation in the ductus arteriosus. J Clin Invest. 2006 Nov;116(11):3026-34.
9. Takayama K, Garcia-Cardena G, Sukhova GK, Comander J, Gimbrone MA Jr, Libby P. Prostaglandin E2 suppresses chemokine production in human macrophages through the EP4 receptor. J Biol Chem. 2002 Nov;277(46):44147-54.
10. Kabashima K, Saji T, Murata T, Nagamachi M, Matsuoka T, Segi E, et al. The prostaglandin receptor EP4 suppresses colitis, mucosal damage and CD4 cell activation in the gut. J Clin Invest. 2002 Apr;109(7):883-93.
11. Nitta M, Hirata I, Toshina K, Murano M, Maemura K, Hamamoto N, et al. Expression of the EP4 prostaglandin E2 receptor subtype with rat dextran sodium sulphate colitis: colitis suppression by a selective agonist, ONO-AE1-329. Scand J Immunol. 2002 Jul;56(1):66-75.
12. Jee WS, Ueno K, Deng YP, Woodbury DM. The effects of prostaglandin E2 in growing rats: increased metaphyseal hard tissue and cortico-endosteal bone formation. Calcif Tissue Int. 1985 Mar;37(2):148-57.
13. Jee WS, Ueno K, Kimmel DB, Woodbury DM, Price P, Woodbury LA. The role of bone cells in increasing metaphyseal hard tissue in rapidly growing rats treated with prostaglandin E2. Bone. 1987;8(3):171-8.
14. Li XJ, Jee WS, Li YL, Patterson-Buckendahl P. Transient effects of subcutaneously administered prostaglandin E2 on cancellous and cortical bone in young adult dogs. Bone. 1990;11(5):353-64.
15. Chyun YS, Raisz LG. Stimulation of bone formation by prostaglandin E2. Prostaglandins. 1984 Jan;27(1):97-103.
16. Nagata T, Kaho K, Nishikawa S, Shinohara H, Wakano Y, Ishida H. Effect of prostaglandin E2 on mineralization of bone nodules formed by fetal rat calvarial cells. Calcif Tissue Int. 1994 Dec;55(6):451-7.
17. Alander CB, Raisz LG. Effects of selective prostaglandins E2 receptor agonists on cultured calvarial murine osteoblastic cells. Prostaglandins Other Lipid Mediat. 2006 Dec;81(3-4):178-83.
18. Shamir D, Keila S, Weinreb M. A selective EP4 receptor antagonist abrogates the stimulation of osteoblast recruitment from bone marrow stromal cells by prostaglandin E2 in vivo and in vitro. Bone. 2004 Jan;34(1):157-62.
19. Weinreb M, Shamir D, Machwate M, Rodan GA, Harada S, Keila S. Prostaglandin E2 (PGE2) increases the number of rat bone marrow osteogenic stromal cells (BMSC) via binding the EP4 receptor, activating sphingosine kinase and inhibiting caspase activity. Prostaglandins Leukot Essent Fatty Acids. 2006 Aug;75(2):81-90.
20. Machwate M, Harada S, Leu CT, Seedor G, Labelle M, Gallant M, et al. Prostaglandin receptor EP(4) mediates the bone anabolic effects of PGE(2). Mol Pharmacol. 2001 Jul;60(1):36-41.
21. Lader CS, Flanagan AM. Prostaglandin E2, interleukin 1alpha, and tumor necrosis factor-alpha increase human osteoclast formation and bone resorption in vitro. Endocrinology. 1998 Jul;139(7):3157-64.
22. Sarrazin P, Bkaily G, Hache R, Patry C, Dumais R, Rocha FA, et al. Characterization of the prostaglandin receptors in human osteoblasts in culture. Prostaglandins Leukot Essent Fatty Acids. 2001 Mar;64(3):203-10.
23. Mano M, Arakawa T, Mano H, Nakagawa M, Kaneda T, Kaneko H, et al. Prostaglandin E2 directly inhibits bone-resorbing activity of isolated mature osteoclasts mainly through the EP4 receptor. Calcif Tissue Int. 2000 Jul;67(1):85-92.
24. Yoshida K, Oida H, Kobayashi T, Maruyama T, Tanaka M, Katayama T, et al. Stimulation of bone formation and prevention of bone loss by prostaglandin E EP4 receptor activation. Proc Natl Acad Sci U S A. 2002 Apr;99(7):4580-5.
25. Iino Y, Toriyama M, Ogawa H, Kawakami M. Cholesteatoma debris as an activator of human monocytes: potentiation of the production of tumor necrosis factor. Acta Otolaryngol. 1990 Nov-Dec;110(5-6):410-5.
26. Xie L, Liu AG, Cui YH, Zhang YP, Liao B, Li NN, et al. Expression profiles of prostaglandin E2 receptor subtypes in aspirin tolerant adult Chinese with chronic rhinosinusitis. Am J Rhinol Allergy. 2015 Sep-Oct;29(5):322-8.
27. Morimoto K, Shirata N, Taketomi Y, Tsuchiya S, Segi-Nishida E, Inazumi T, et al. Prostaglandin E2-EP3 signaling induces inflammatory swelling by mast cell activation. J Immunol. 2014 Feb;192(3):1130-7.
|
|
|||||||||||||||||||||||||||||||||||||||||||||







