 |
 |
- Search
AbstractObjectivesFacial nerve monitoring (FNM) can be used to identify the facial nerve, to obtain information regarding its course, and to evaluate its status during parotidectomy. However, there has been disagreement regarding the efficacy of FNM in reducing the incidence of facial nerve palsy during parotid surgery. Therefore, instead of using electromyography (EMG) to identify the location and state of the facial nerve, we applied an intraoperative neuromonitoring (IONM) system using a surface pressure sensor to detect facial muscle twitching. The objective of this study was to investigate the feasibility of using the IONM system with a surface pressure sensor to detect facial muscle twitching during parotidectomy.
MethodsWe evaluated the stimulus thresholds for the detection of muscle twitching in the orbicularis oris and orbicularis oculi, as well as the amplitude and latency of EMG and the surface pressure sensor in 13 facial nerves of seven rabbits, using the same stimulus intensity.
Facial nerve palsy (FNP) is an immediate and potentially serious complication of parotid or otologic surgery. In addition to causing cosmetic problems, FNP can also lead to functional impairment, affecting patients’ quality of life and potentially resulting in expensive medical litigation [1,2]. Most cases of facial nerve damage are caused by stretching, entrapment, compression, thermal injury, or ischemia [3]. Hitherto, transient FNP has been reported in approximately 65% of parotidectomy patients, and permanent facial weakness has been reported in 4% to 7% of patients [4-9]. Facial nerve monitoring (FNM) is a procedure that can be used to identify the facial nerve, obtain information regarding the course of the facial nerve, and evaluate the status of the facial nerve in patients undergoing parotidectomy [10]. Intraoperative FNM is an adjunctive method that can be used by surgeons to ensure the functional preservation of the facial nerve during parotid, neurotologic, and skull-base surgery. It has been reported that in the United States, 60% of surgeons used FNM during parotidectomy, but 40% did not [10]. A recent meta-analysis showed a lower occurrence of immediate FNP after parotidectomy performed using FNM than after parotidectomy performed without using FNM (22.5% vs. 34.2%, respectively). However, no significant difference in the occurrence of permanent FNP was evident between the FNM group and the non-FNM group (3.9% vs. 7.1%, respectively) [11]. These results are consistent with those of previous studies [4,5,9]. In light of these findings, the need for intraoperative FNM during parotidectomy is controversial.
In addition, a study reported that only 16% of patients showed abnormal electromyography (EMG) results during parotid surgery; however, 65% of the patients in that study had postoperative transient or permanent FNP. It was concluded that FNM using EMG could not predict postoperative neurological outcomes accurately [4]. Both false-negative and false-positive responses may occur when monitoring patients for potential facial nerve damage. The discrepancy between intraoperative EMG findings and postoperative facial nerve function can be explained by several factors. First, the distal segment of the transected facial nerve can be stimulated by electrical pulses; therefore, the surgeon may not be aware of the resultant nerve damage. Moreover, the EMG findings of healthy facial nerves may be affected by the depth of anesthesia, neuromuscular blockers, and/or electrocautery. Another disadvantage of intraoperative EMG monitoring of the facial nerves is the need to insert an electrode into the facial muscles to obtain EMG recordings. This insertion may cause infection, inflammation, bleeding, and facial edema around the eye and mouth [9].
Compared with EMG measurements, which reflect electrical activity in muscles, measuring vibrations or skin surface pressure around the muscles, as reported in previous studies [12], does not require the insertion of a needle electrode and might be less strongly influenced by electrocauterization. Therefore, a relatively novel intraoperative neuromonitoring (IONM) system that analyzes muscle twitching using a skin surface pressure sensor was proposed to evaluate the status of the nerves instead of recording EMG responses. The aim of this study was to investigate the feasibility and reliability of the IONM system using a skin surface pressure sensor to detect facial muscle twitching during parotid surgery in a rabbit model.
The experimental protocol was approved by the Institutional Animal Care and Use Committee (No. PNUYH-2018-51). The experiment was performed on thirteen facial nerves in seven female rabbits. The rabbits were sedated using an intramuscular injection of ketamine hydrochloride (10 mg/kg) and xylazine (2 mg/kg), and anesthesia was maintained by inhaled 3% isoflurane. After the rabbits were anesthetized, they were placed in a lateral decubitus position. Both sides of the facial region were shaved, and a skin incision was made, followed by the dissection of the buccal and zygomatic branches of the facial nerve.
The amplitudes and latencies of needle EMG and surface pressure sensor were recorded at every step. All rabbits were monitored using a nerve integrity monitor (NIM-Response 3.0 System; Medtronic Xomed, Jacksonville, FL, USA), and a hand-made surface pressure sensor monitor. A pair of EMG needle electrodes was inserted into the orbicularis oris and orbicularis oculi muscles to perform the EMG. Simultaneously, a surface pressure sensor was attached to the skin above the orbicularis oris and orbicularis oculi muscles (Fig. 1). After the end of the experiment, the anesthetized rabbits were euthanized.
The displayed stimulation threshold of EMG was the stimulus value at which the response was first evident. The mean amplitudes of needle EMG and surface pressure sensor for each stimulus value were compared (Fig. 2). The stimulus thresholds of EMG were analyzed to detect facial muscle movement, and the amplitudes and latencies of needle EMG and surface pressure sensor were measured in the thirteen facial nerves of seven rabbits.
As mentioned in the previous paper, the surface pressure sensor utilizes the piezo-electric effect, which is responsible for its characteristic diagnostic ability (Fig. 1) [12]. As it is a patch-type device, it is attached to the skin. When attached to the skin, it measures the surface pressure caused by twitching of the facial muscle due to facial nerve stimulation. Additional supplementary material is available in the online version of the article (Supplementary Material 1, video clip).
The stimulus threshold, amplitude, and latency of needle EMG and the surface pressure sensor were evaluated in the buccal branch of 13 facial nerves (Table 1, Fig. 2). When the nerves were stimulated by a current of 0.02 mA, the amplitude of the needle EMG and surface pressure sensor at the orbicularis oris muscle was 125.50±162.63 μV and 144.00±154.97 mV, respectively, and their latency was 3.75±0.00 ms and 26.00±5.65 ms, respectively, in two buccal branches of the facial nerves. When stimulated by a current of 0.03 mA, the amplitude of the needle EMG and surface pressure sensor at the orbicularis oculi muscle was 209.50±160.51 μV and 68.00±19.79 mV, respectively, and their latency was 4.75±0.35 ms and 25.50±7.77 ms, respectively, in two zygomatic branches of the facial nerves. Both the needle EMG and surface pressure sensor were able to detect facial muscle twitching in response to a stimulation of 0.1 mA in all 13 facial nerves. As shown in Tables 1 and 2, the stimulus thresholds did not differ significantly between the needle EMG and the surface pressure sensor.
The mean amplitude of the needle EMG and surface pressure sensor for the buccal branch of the facial nerve was 691.35±622.01 μV and 301.90±166.77 mV, respectively. The mean latency of the needle EMG and surface pressure sensor for the buccal branch of the facial nerve was 3.90±0.33 ms and 24.32±4.73 ms, respectively. The mean amplitude of the needle EMG and surface pressure sensor for the zygomatic branch of the facial nerve was 537.09±361.88 μV and 375.56±257.84 mV, respectively. The mean latency of the needle EMG and surface pressure sensor for the zygomatic branch of the facial nerve was 4.04±0.59 ms and 23.31±4.98 ms, respectively. As shown in Tables 1 and 2, the differences in amplitude and latency between the needle EMG and surface pressure sensor were significantly different, depending on the stimulus value (P<0.001 and P<0.001, respectively). A direct comparison of the difference in amplitude was not significant due to the differences in the measurement units. The mean latency difference was approximately 20 ms, which is clinically significant during parotidectomy.
FNP after surgery is a complication that surgeons should strive to avoid. To some extent, FNP can be avoided by adequate knowledge of the facial anatomy, which is helpful for direct identification of the facial nerve. However, the location of the facial nerve can be altered in the presence of a large tumor, in cases of reoperation, or in the presence of severe adhesions due to inflammation, which might render identification of the facial nerve based on knowledge of the facial anatomy alone difficult [8,13]. In such cases, FNM can prove to be of considerable value for the surgeon as a way to locate the path of the facial nerve. During parotid surgery, if the surgeon has difficulty in localizing the nerve, a nerve probe is used to identify the main trunk of the facial nerve, to identify peripheral branches during retrograde facial nerve dissection, to evaluate the status of the nerve, and to predict postoperative facial nerve function [3]. As the probe helps to identify the nerve, the operation time is shortened and the safety of the patient is enhanced [6,9,10]. However, whether FNM can help reduce the incidence of FNP during parotid surgery remains controversial.
Lopez et al. [14] reported that the use of FNM reduced the incidence of both temporary and permanent FNP. Makeieff et al. [15] also documented that patients in whom FNM was used showed a lower proportion of FNP than those in whom FNM was not used. However, reasons to disfavor the use of FNM include economic constraints and the time required for set-up. Terrell et al. [16] showed that FNM did not reduce the operation time. Witt reported that no association was found between the use of FNM and a decrease in postoperative temporary FNP. Meier et al. [4] reported a discrepancy between postoperative abnormal EMG results and postoperative functional integrity as determined by FNM. Identifying the facial nerve using IONM by EMG during parotidectomy is helpful; however, the accuracy of prediction of postoperative nerve function using this method and the degree to which it can prevent intraoperative nerve injury remains controversial [5,6,11].
EMG monitoring can be missed or misinterpreted due to the effects of muscle relaxants, inflammation, nerve damage caused due to invasion by cancer, electrocauterization, and/or cold-water stimulation, as well as due to errors in the EMG monitor setup [4,6,17-19]. FNM can often provide false-positive responses. It is important to distinguish between actual EMG signals and artifacts resulting from contact with surgical instruments in the surgical field during IONM [3]. The EMG waveform can vary depending on the situation. Furthermore, even if the proximal part of the nerve is damaged, peripheral muscle activity can be caused by the stimuli discharged during EMG [4,20]. In addition, needle electrodes can cause infection, bleeding, and/or postoperative pain, and can injure the surgeon during insertion and removal of the needle electrodes [3]. A study reported three cases of facial burns at needle electrode insertion sites associated with a technical error in the monitoring devices [21]. If an abnormal signal is seen on the IONM system, facial muscle twitching is checked using a finger to confirm that the function of the facial nerve is intact. By using an objective sensor instead of a finger, the movement of the muscle can be detected, and the location and status of the nerve can be identified more accurately.
A piezoelectric pressure sensor was modified to measure changes in skin surface pressure and to identify facial muscle movement [12,22]. The objective of the present study was to determine the feasibility and reliability of FNM using a surface pressure sensor in a rabbit model. The surface pressure sensor and EMG reacted to a stimulus of 0.1 mA, indicating that there was no difference in the stimulus threshold between the surface pressure sensor and EMG in the identification of facial muscle movement. As the pressure sensor is a patch-type device, the surgeon is required to attach the sensor to the skin, with no need to insert a needle.
The surface pressure sensor is designed to overcome the evident drawbacks of EMG during parotid surgery. EMG identifies muscle movement by measuring the difference in the action potential between the two electrodes. Therefore, if the position of even one electrode changes due to a postural shift during parotidectomy, muscle movement cannot be precisely assessed by EMG. However, a patch-type device can assess muscle twitching by surface contact with a small part of the skin. Therefore, a piezoelectric pressure sensor is less sensitive to postural changes than EMG measurements. Since no electrode is inserted into the muscle to measure the amplitude of EMG during surgery, this device is noninvasive and does not pose any risks of bleeding, infection, or pain.
There are some limitations to this study. Since the sample size was small, and the study was conducted on animals, further large-scale human studies including a larger sample size are warranted to determine the feasibility and safety of using a surface pressure sensor during parotidectomy in humans. In conclusion, the use of a piezoelectric surface pressure sensor that detects muscle twitching is a noninvasive, reliable, and feasible approach, as there was no difference in the stimulus threshold for the detection of muscle twitching between EMG and the surface pressure sensor. Therefore, an IONM system that uses a surface pressure sensor to measure facial muscle twitching is an alternative to EMG for analyzing the status of the facial nerve.
▪ The use of a surface pressure sensor and electromyography in facial nerve monitoring during parotidectomy was compared.
▪ The stimulus thresholds did not significantly differ between the system with a surface pressure sensor and electromyography.
▪ The application of intraoperative neuromonitoring with a surface pressure sensor during parotidectomy is noninvasive, reliable, and feasible.
NotesAUTHOR CONTRIBUTIONS Conceptualization: BJL. Data curation: YIC, JWL, DHP, SWC, HBK, HJP. Formal analysis: SCS, HKK. Methodology: JHR. Project administration: JCL. Visualization: JHR. Writing–original draft: ESS. Writing–review & editing: ESS, BJL. SUPPLEMENTARY MATERIALSSupplementary materials can be found via https://doi.org/10.21053/ceo.2019.01900.
Fig. 1.Surface pressure sensor and surgical field. (A) Photograph of the patch-type surface pressure sensor. (B) Photograph of the surgical field in the rabbit, demonstrating facial nerve dissection. Black arrow, buccal branch of the facial nerve; white arrow, zygomatic branch of the facial nerve. (C) Stimulation of the buccal branch of the facial nerve using the nerve probe. (D) Illustration of a pair of electromyography needle electrodes inserted into the orbicularis oris and orbicularis oculi muscles. Simultaneously, a surface pressure sensor was attached to the skin above the orbicularis oris and oculi muscles. 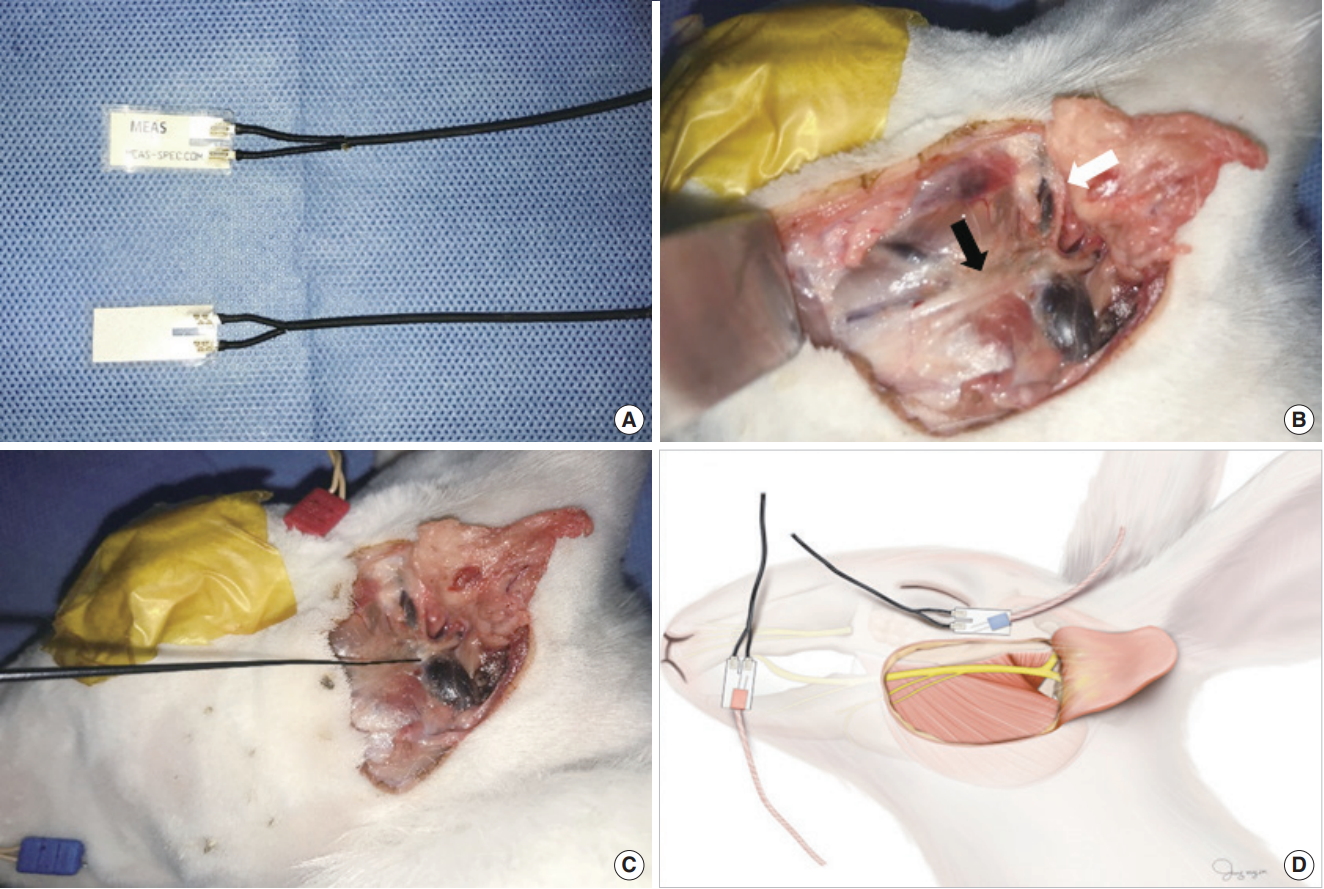
Fig. 2.Electromyography (EMG) and surface pressure sensor recordings showing the amplitude and latency of EMG and the pressure sensor for a stimulation of 0.3 mA using the nerve integrity monitor 3.0 system in a rabbit. (A) Pressure sensor signal. (B) EMG signal. 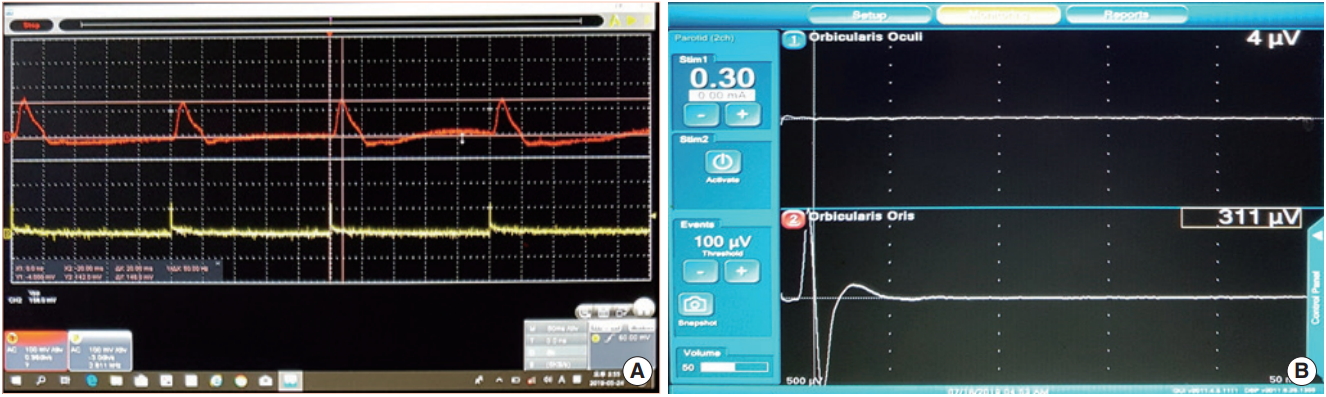
Table 1.Analysis of the amplitude and latency of needle EMG and the surface pressure sensor at the orbicularis oris muscle depending on the intensity of the facial nerve stimulus under intermittent intraoperative neuromonitoring in rabbits Table 2.Analysis of the amplitude and latency of needle EMG and the surface pressure sensor at the orbicularis oculi muscle depending on the intensity of the facial nerve stimulus under intermittent intraoperative neuromonitoring in rabbits REFERENCES1. Ryzenman JM, Pensak ML, Tew JM Jr. Facial paralysis and surgical rehabilitation: a quality of life analysis in a cohort of 1,595 patients after acoustic neuroma surgery. Otol Neurotol. 2005 May;26(3):516-21.
2. Lydiatt DD. Medical malpractice and facial nerve paralysis. Arch Otolaryngol Head Neck Surg. 2003 Jan;129(1):50-3.
3. Eisele DW, Wang SJ, Orloff LA. Electrophysiologic facial nerve monitoring during parotidectomy. Head Neck. 2010 Mar;32(3):399-405.
4. Meier JD, Wenig BL, Manders EC, Nenonene EK. Continuous intraoperative facial nerve monitoring in predicting postoperative injury during parotidectomy. Laryngoscope. 2006 Sep;116(9):1569-72.
5. Laccourreye H, Laccourreye O, Cauchois R, Jouffre V, Menard M, Brasnu D. Total conservative parotidectomy for primary benign pleomorphic adenoma of the parotid gland: a 25-year experience with 229 patients. Laryngoscope. 1994 Dec;104(12):1487-94.
6. Dulguerov P, Marchal F, Lehmann W. Postparotidectomy facial nerve paralysis: possible etiologic factors and results with routine facial nerve monitoring. Laryngoscope. 1999 May;109(5):754-62.
7. Witt RL. Facial nerve function after partial superficial parotidectomy: an 11-year review (1987-1997). Otolaryngol Head Neck Surg. 1999 Sep;121(3):210-3.
8. Upton DC, McNamar JP, Connor NP, Harari PM, Hartig GK. Parotidectomy: ten-year review of 237 cases at a single institution. Otolaryngol Head Neck Surg. 2007 May;136(5):788-92.
9. Grosheva M, Klussmann JP, Grimminger C, Wittekindt C, Beutner D, Pantel M, et al. Electromyographic facial nerve monitoring during parotidectomy for benign lesions does not improve the outcome of postoperative facial nerve function: a prospective two-center trial. Laryngoscope. 2009 Dec;119(12):2299-305.
10. Lowry TR, Gal TJ, Brennan JA. Patterns of use of facial nerve monitoring during parotid gland surgery. Otolaryngol Head Neck Surg. 2005 Sep;133(3):313-8.
11. Sood AJ, Houlton JJ, Nguyen SA, Gillespie MB. Facial nerve monitoring during parotidectomy: a systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2015 Apr;152(4):631-7.
12. Sung ES, Lee JC, Shin SC, Kwon HG, Kim MS, Kim DJ, et al. Development of a novel intraoperative neuromonitoring system using a surface pressure sensor to detect muscle movement: a rabbit model study. Clin Exp Otorhinolaryngol. 2019 May;12(2):217-23.
13. Olsen KD, Daube JR. Intraoperative monitoring of the facial nerve: an aid in the management of parotid gland recurrent pleomorphic adenomas. Laryngoscope. 1994 Feb;104(2):229-32.
14. Lopez M, Quer M, Leon X, Orus C, Recher K, Verges J. Usefulness of facial nerve monitoring during parotidectomy. Acta Otorrinolaringol Esp. 2001 Jun-Jul;52(5):418-21.
15. Makeieff M, Venail F, Cartier C, Garrel R, Crampette L, Guerrier B. Continuous facial nerve monitoring during pleomorphic adenoma recurrence surgery. Laryngoscope. 2005 Jul;115(7):1310-4.
16. Terrell JE, Kileny PR, Yian C, Esclamado RM, Bradford CR, Pillsbury MS, et al. Clinical outcome of continuous facial nerve monitoring during primary parotidectomy. Arch Otolaryngol Head Neck Surg. 1997 Oct;123(10):1081-7.
17. Grosheva M, Guntinas-Lichius O. Significance of electromyography to predict and evaluate facial function outcome after acute peripheral facial palsy. Eur Arch Otorhinolaryngol. 2007 Dec;264(12):1491-5.
18. Anon JB, Lipman SP, Guelcher RT, Sibly DA, Thumfart W. Monitoring the facial nerve during parotidectomy. Arch Otolaryngol Head Neck Surg. 1991 Dec;117(12):1420.
19. Empis de Vendin O, Schmartz D, Brunaud L, Fuchs-Buder T. Recurrent laryngeal nerve monitoring and rocuronium: a selective sugammadex reversal protocol. World J Surg. 2017 Sep;41(9):2298-303.
20. Harper CM, Daube JR. Facial nerve electromyography and other cranial nerve monitoring. J Clin Neurophysiol. 1998 May;15(3):206-16.
|
|
|||||||||||||||||||||||||||||||||||||||||||







