 |
 |
- Search
AbstractObjectives. The recent expansion of eligibility for cochlear implantation (CI) by the U.S. Food and Drug Administration (FDA) to include infants as young as 9 months has reignited debates concerning the clinically appropriate cut-off age for pediatric CI. Our study compared the early postoperative trajectories of receptive and expressive language development in children who received CI before 9 months of age with those who received it between 9 and 12 months. This study involved a unique pediatric cohort with documented etiology, where the timing of CI was based on objective criteria and efforts were made to minimize the influence of parental socioeconomic status.
Methods. A retrospective review of 98 pediatric implantees recruited at a tertiary referral center was conducted. The timing of CI was based on auditory and language criteria focused on the extent of delay corresponding to the bottom 1st percentile of language development among age-matched controls, with patients categorized into very early (CI at <9 months), early (CI at 9ŌĆō12 months) and delayed (CI at 12ŌĆō18 months) CI groups. Postoperative receptive/expressive language development was assessed using the Sequenced Language Scale for Infants receptive and expressive standardized scores and percentiles.
Results. Only the very early CI group showed significant improvements in receptive language starting at 3 months post-CI, aligning with normal-hearing peers by 9 months and maintaining this level until age 2 years. During this period (<2 years), all improvements were more pronounced in receptive language than in expressive language.
Conclusion. CI before 9 months of age significantly improved receptive language development compared to later CI, with improvements sustained at least up to the age of 2. This study supports the consideration of earlier CI, beyond pediatric Food and Drug Administration labeling criteria (>9 months), in children with profound deafness who have a clear deafness etiology and language development delays (<1st percentile).
The recent expansion of the U.S. Food and Drug Administration (FDA) criteria for cochlear implantation (CI) to include infants as young as 9 months [1,2] has reignited long-standing debates about the optimal age for pediatric CI. A particularly recent retrospective study from the United States showed that children who received CI before 9 months of age exhibited superior receptive and expressive language development after an average follow-up of 7.4 years, compared to those who received CI between 9 and 12 months [3]. There is a growing body of evidence supporting the lasting benefits of CI at increasingly younger ages [4-7], and little research suggests that the advantages of early CI diminish when performed before 9 months [3,8-10]. We recommend fitting hearing aids by no later than 6 months of age, or even earlier for newborns with significant hearing loss, in accordance with the Joint Committee on Infant Hearing (JCIH) guidelines, which recommend timely intervention by 6 months of age. Extending this rationale, it may be beneficial, or even essential, for infants with profound, irreversible sensorineural hearing loss to receive CI surgery before reaching 9 months of age. Such an approach is in line with the JCIHŌĆÖs recommendations for early intervention and has the potential to maximize the benefits for language development.
However, while hearing aids are widely accepted, there are concerns regarding CI at a very early age, such as before 9 months. These concerns include the potential side effects of general anesthesia in infants, perioperative risks associated with such an early age, and the difficulties in accurately assessing hearing status in very young infants. However, recent reports suggest that the side effects of general anesthesia and surgery in very young children are not considered significant issues [7,11]. Nevertheless, before routinely implementing CI in infants, several issues need further clarification. First, the existing literature does not provide adequate information about the causes of hearing loss or the degree of speech development delay prior to CI in the very early CI group, such as those younger than 9 months, compared to those who receive the implant later. Most studies have focused on speech development outcomes after surgery, without offering detailed accounts of pre-CI language development or the criteria for performing CI before 9 months or between 9 and 12 months of age. Second, it is unclear whether the benefits of very early CI are immediately apparent, even within the first year following the procedure, in terms of receptive and expressive language development. Although children who received their implants and activation before 9 months of age have been reported to exhibit rapid auditory skill development, reaching the expected performance level of normal-hearing peers by the age of 2 [10], the early trajectory of receptive and expressive language development in the first 1ŌĆō2 years post-implantation for the under-9-months groupŌĆöwhich could reflect the direct impact of CI without confounding factorsŌĆöcompared to long-term outcomes, remains largely unexplored.
Given this, we aim to provide comparative data on the early speech development of children who received CI before 9 months of age versus those who received it between 9 and 12 months. Our pediatric cohort consisted of cases where the genetic and radiologic etiology was clearly documented in over 90% of instances. Furthermore, the decision regarding the timing of CI is based solely on objective criteria that focus on the extent of language development delay compared to age-matched controls. By adopting this methodology, we strive to minimize the potential influence of the infantŌĆÖs parentsŌĆÖ socioeconomic status (SES) or their enthusiasm for auditory rehabilitation on the timing of CI. The South Korean healthcare insurance system has played a significant role in facilitating this approach. This method should enable us to accurately assess the impact of the age at CI on language development during the first 1ŌĆō2 years postoperatively.
We retrospectively reviewed the medical records of 98 pediatric patients (59 boys and 39 girls). who underwent unilateral or bilateral, simultaneous, or sequential CI under 3 years of age between July 2018 and April 2022 by a single surgeon. Decision of the implantation timing was determined and planned according to two diagnostic criteria: (1) when the average of auditory brain-stem response (ABR) and/or auditory steady-state response (ASSR) thresholds was same or worse than 90 dB bilaterally at least twice or more, (2) when a receptive score of speech evaluation test was delayed by 5 months or more than compared to that of normal children of equivalent age even after 3 months of hearing aid rehabilitation and rigorous speech therapy under a normal cognitive status. During the age period from 6 to 21 months, a language delay of 5 months or more would place them in the bottom one percentile (<1%) of language development levels among age-matched normal hearing peers based on the Sequenced Language Scale for Infants (SELSI) test results (Supplementary Table 1) [12-14]. We also obtained behavioral thresholds at least for the 1 kHz pure tone from all the candidates and tried to measure behavioral thresholds from as many frequencies as possible. If the etiology of hearing loss identified through genetic testing that would be described below or imaging test did not match the audiological test results, we repeated ABR/ASSR.
In the case of children who meet both criteria, CI was performed at as early as 5 months of age. In contrast, when the hearing threshold was better (or lower) than 90 dB, auditory rehabilitation with hearing aids and periodic speech evaluation was mainly applied at least for up to 12 months of age. After then, CI was also considered if receptive language got delayed by more than 5 months compared to the age-matched control group. All ninety-eight pediatric patients had worn hearing aids for at least 3 months before the first CI surgery and were implanted with devices from Cochlear Ltd. Depending on the average age at first CI, pediatric implantees were classified as the very early CI group (<9 months of age: group A), the early CI group (9ŌĆō12 months: group B) and the delayed CI group (12ŌĆō18 months: group C). In all CIs for both the very early CI group and the early CI group, the same type of electrode, the slim modiolar electrode (CI532 or CI632), was used.
The medical records, audiological and radiological examinations, and the etiologic configurations were retrospectively reviewed. As mentioned above, audiological evaluations were examined with ABRT and ASSR. Congenital cytomegalovirus (CMV) infection was initially inspected in all cohorts through CMV viral culture and CMV-polymerase chain reaction testing. Radiological tests were conducted with temporal bone computed tomography and internal auditory canal magnetic resonance imaging for the possible diagnosis of inner ear anomalies or lesions of auditory and central nerve structures. Pediatric patients diagnosed with meningitis, global developmental delay, and cognitive delay as evaluated by Bayley Scales of Infant and Toddler Development Screening Test (BSID version 4) [15] were excluded in this study.
Given the retrospective nature of the chart review, this study was approved, and informed consent was waived by the Institutional Review Board of the Clinical Research Institute (No. B-2302-813-102). All protocols and procedures were conducted in accordance with the Declaration of Helsinki.
Speech perception performance was assessed by using both SELSI [12-14,16] and Receptive and Expressive Vocabulary Test (REVT) [17] pre- and postoperatively according to the childrenŌĆÖs chronological age. The SELSI and REVT tests are Korean version of screening tools for the evaluation of language and vocabulary development skills and have confirmed high consistency and reliability among preschool infants and children in Korea [12-14,18]. Especially, both tests include questions which can evaluate the degree of receptive and expressive language development in forms of raw scores and percentiles. REVT can be performed on children aged Ōēź2 years and SELSI on younger children, which gives an opportunity to measure speech development in a broader age range [16,17]. Numerous studies have detailed the extent of speech and language development using the SELSI and REVT tests [12,18-20]. Speech evaluation was implemented by professional speech and language pathologists during each speech evaluation session preoperatively, 3, 6, 9, and 12 months postoperatively and at 24 months of age. Specifically, trends in receptive and expressive language development between groups at the same time point for one year after surgery were compared.
According to our institutionŌĆÖs diagnostic pipeline for congenital deafness, molecular genetic testing was performed in all cohorts. First, genomic DNA samples were extracted from the peripheral blood [21]. molecular genetic testing was conducted with the following processes, which have been previously described in our papers: initial screening test with U-Top Genotyping Screening Kit (SeaSun Biomaterials), followed by a validation process using direct Sanger sequencing (Macrogen Inc.) [22-24]. Some patients with specific audiological phenotypes such as ski-slope type hearing loss or radiological findings including enlarged vestibular aqueduct underwent direct Sanger sequencing, as appropriate [25]. Deafness panel sequencing (TES-129) [26] or exome sequencing [27] were conducted in patients who were not diagnosed after screening tests.
All children were thoroughly monitored with postoperative surgical complications at the outpatient clinic. Complications included surgical site infection, swelling or dehiscence after implantation surgery.
All the data are presented as mean┬▒standard deviation. All statistical analyses were performed and depicted using the IBM SPSS software (ver. 25; IBM Corp.) and the GraphPad Prism software (ver. 9.0.0; GraphPad Software Inc.). Percentiles of receptive and expressive SELSI and REVT tests were compared pairwise between groups at each postoperative period with a Mann-Whitney U-test. A simple correlation analysis was used to evaluate relationship between age at implantation and percentile scores of SELSI receptive and expressive results within non-cochlear nerve deficiency (non-CND) patients.
Based on the decision strategy for CI timing at our institute, we provided the age distribution at the time of CI for our entire pediatric cohort (n=98), all of whom underwent their first CI before the age of 3 years. The average age at CI was 12.5┬▒7.1 months (Fig. 1). Specifically, the average age at CI for the very early CI group (<9 months: group A, n=41) was 7.1┬▒0.7 months, for the early CI group (9 to Ōēż12 months: group B, n=25) was 10.4┬▒1.2 months, and for the delayed CI group (12 to Ōēż18 months: group C, n=14) was 14.6┬▒1.4 months. Eighty-three operations (84.7%) were bilateral simultaneous, 3 (3.1%) were bilateral sequential, and 12 (12.2%) were unilateral. There was no statistically significant difference in the rate of bilateral CIs between the very early CI group and the early CI group. However, the rate of bilateral CIs in the very early CI group was significantly higher compared to the delayed CI group (P=0.0139, FisherŌĆÖs exact test).
Compared to their normal age equivalents, the average delay in preoperative receptive speech scores for each group at the time of CI was 5.2┬▒1.0 months, 6.5┬▒1.6 months, and 4.7┬▒2.6 months, respectively. The average ratio of the immediate preoperative speech-equivalent age to the actual age for each of the three groups was 0.26┬▒0.12, 0.37┬▒0.17, and 0.67┬▒0.17, respectively. This suggests that the groups who received implants earlier exhibited a greater degree of delayed speech development. At the time of this retrospective study, the average postoperative follow-up period for each group was 14.2┬▒3.1 months, 12.9┬▒1.5 months, and 14.0┬▒4.7 months, respectively.
The etiology was confirmed in 86 of 98 cases in our pediatric cohort, while that of 12 cases remained unclarified (Table 1). Specifically, in the very early CI group (<9 months: group A), CND was the most frequent cause, constituting 24.4% of this group (n=10), followed by DFNB1 due to GJB2 variants (n=7), DFNB4 due to SLC26A4 variants (n =5) and DFNB9 due to OTOF variants (n =3). In the early CI group (9ŌĆō12 months: group B), DFNB1 due to GJB2 variants was the most frequent causative etiology (n=7), followed by CND (n=5), and DFNB4 due to SLC26A4 and DFNB9 due to an OTOF variant (each n=3). In the delayed CI group (12ŌĆō18 months: group C), DFNB4 due to SLC26A4 constituted the most common etiology (n=7). A comparison of baseline characteristics is shown in Table 1. When CND was excluded from the cohort, the average age at CI was 7.3┬▒0.8, 10.7┬▒1.2, and 15.2┬▒2.0 months old in the very early CI (n =31), early CI (n =20), and late CI (n =13) groups, respectively. All CND patients were diagnosed according to the diagnostic pipeline of our institution prior to CI surgery, and surgery was performed after sufficient preoperative counseling, following the routine protocol.
Among the non-CND subgroups, the very early CI group exhibited the poorest ABR and ASSR thresholds immediately before CI, with both ears showing an ABR threshold of 98.7┬▒3.4 dB and ASSR thresholds of 96.6┬▒3.9 dB for the better ear and 97.8┬▒3.3 dB for the worse ear. The average ABR thresholds for the better and worse ears were 92.8┬▒13.5 dB and 98.0┬▒5.2 dB, respectively, for the early CI group, and 82.0┬▒20.3 dB and 97.0┬▒6.7 dB, respectively, for the delayed CI group. The average ASSR thresholds followed a similar pattern; for the early CI group, they were 89.1┬▒11.9 dB for the better ear and 96.1┬▒6.7 dB for the worse ear, while for the late CI group, they were 79.6┬▒14.5 dB for the better ear and 91.8┬▒7.0 dB for the worse ear.
When the better ears were compared, the very early CI group (group A) showed significantly worse ASSR thresholds than the other two groups (groups B and C) (Mann-Whitney U-test, P=0.010 and <0.001, respectively). The ABR thresholds significantly differed only between Groups A and C (Mann-Whitney U-test, P=0.010). There was no significant difference in the ABR and ASSR thresholds between Groups B and C (Mann-Whitney U-test) (Supplementary Table 2).
When analyzing the outcomes of recipients diagnosed with CND, the CND group exhibited significantly poorer results during most postoperative periods in both receptive and expressive scores (Supplementary Fig. 1, Supplementary Table 3). Given the substantially negative impact on the overall postoperative speech evaluation results, which diluted the effect of early CI, our subsequent analysis excluded patients with CND. We then compared the three different non-CND subgroups as previously mentioned.
Both receptive and expressive scores from the SELSI were evaluated using percentile scores throughout the first post-implant year and at the age of 2 years. For receptive percentiles, the very early CI group demonstrated immediately higher percentiles from 3 months postoperatively and continued to outperform the other two non-CND groups when compared over the 12-month postoperative period; statistical significance was observed at the postoperative 3-, 9-, and 12-month intervals only between the very early and early CI groups (Table 2). Regarding the SELSI expressive scores, there was no significant difference in the improvement of percentile scores among all three groups until 9 months post-CI. However, from 9 months onward, the very early CI group maintained a trend of improvement in percentiles, while the other two groups did not, although this trend did not reach statistical significance between groups for the SELSI expressive score results (Table 2). When speech scores were compared at the age of 2 years, the very early CI group again exhibited higher SELSI receptive percentile scores than the other two groups, with statistical significance observed between the very early and early CI groups only (P=0.041, Mann-Whitney U-test). For the SELSI expressive scores, no significant differences were found among the three groups (Table 2).
Additionally, correlation analysis between SELSI receptive/expressive scores and age at CI, ranging from 5 to at least 24 months, revealed overall negative correlations at every postoperative period. This suggests that the benefits of earlier CI are more pronounced for younger children, especially those under 9 months of age. The P-values were 0.186, 0.004, 0.024, 0.052, and <0.001 for receptive scores at postoperative months 3, 6, 9, 12, and at 24 months, respectively; for expressive scores, the P-values were 0.042, 0.045, 0.042, 0.347, and 0.952 at the same respective postoperative intervals (Fig. 2A and B). Additionally, the receptive scores at the 24-month period showed significantly negative correlations with younger age, whereas expressive scores did not demonstrate this trend (Fig. 2C).
Postoperative surgical complications were observed in six out of 98 patients (6.1%). Major complications, such as local flap infection and skin necrosis, occurred in two cases within the very early CI group, leading to the need for device removal and subsequent reimplantation. However, the incidence of major complications did not significantly differ between the very early CI group and the other groups (P=0.1725, FisherŌĆÖs exact test). The remaining four cases involved minor complications, including swelling, redness, and dehiscence at the surgical site. Notably, there were no complications in the early CI group, while two instances of minor complications occurred in both the delayed CI group and the group implanted after 18 months. None of these minor complications required additional revision surgery.
Children with congenital severe to profound hearing loss are now being identified at increasingly younger ages, a trend likely linked to the global, gradual implementation of newborn hearing screening programs. This earlier detection naturally results in the earlier fitting of hearing aids and is anticipated to lower the age at which CI is performed. Numerous studies have demonstrated that performing CI on children with profound hearing loss before the age of 12 months is as safe as CI at later ages. It does not result in a higher rate of complications and is more efficacious [28-31].
The gradual and prolonged reduction of the minimum age for pediatric CI by the U.S. FDAŌĆöfrom 12 months to 9 monthsŌĆöover a span of 20 years underscores the complexity of this decision, given the rigorous evidence required. Factors that have influenced the move toward earlier CI include early fitting of hearing aids, confirmed profound hearing loss, higher SES, and the presence of specific genetic markers such as the GJB2 variant [32]. Financial and rehabilitative commitments, especially in countries lacking governmental support for CI, often restrict access to the procedure to more affluent families. Additionally, the challenge of obtaining reliable auditory responses from very young infants adds complexity to early CI decisions. This is further complicated by the uncertainty surrounding the etiology of hearing loss, which can lead to hesitancy among guardians and surgeons. A more accurate evaluation of the effects of very early CI would be best achieved through a cohort study designed to minimize biases and hesitancy.
Our center employs a comprehensive set of criteria to determine the appropriate timing for CI in pre-lingual pediatric candidates. Initially, we conduct genetic testing and developmental assessments, including the BSID-4, and rigorously evaluate the progress of language development with the aid of hearing aids and intensive speech therapy. CI is considered for infants who do not have significant cognitive delays and who demonstrate a substantial delay in language development, defined as being 5 months or more behind their age-matched peers. This method ensures that decisions regarding the timing of CI are made objectively. We have also analyzed CI outcomes, excluding children with CND, to isolate the effect of the age at surgery on the effectiveness of CI. Additionally, in South Korea, bilateral CI is covered by national insurance for infants under 12 months of age, provided they meet the hearing threshold criterion (a hearing loss worse than 90 dB) and exhibit an evident delay in language development. This eliminates SES as a factor in CI decisionmaking. Consequently, our study was able to assess the impact of CI timing without parental influence, offering a clear perspective on the advantages of early CI.
Based on the CI candidacy criteria at our center, we have observed a higher proportion of infants with profound deafness receiving CIs before the age of 9 months, as opposed to between 9 and 12 months. Specifically, a significantly larger number of infants with profound hearing loss underwent CI surgery before reaching 9 months of age (n=41) compared to those who underwent CI between 9 and 12 months (n=25). Consequently, comparing the surgical outcomes of these very early implantees with those who underwent CI between 9 and 12 months is of even greater significance.
Our study warrants special attention as it provides an early trajectory of receptive and expressive speech development in CI recipients, particularly among those implanted very early (CI at <9 months of age) compared to early implantees (CI at 9ŌĆō12 months of age) and those with delayed implantation (CI at 12ŌĆō18 months of age). We discovered that infants who received implants before 9 months exhibited superior receptive language development compared to those implanted between 9 and 12 months, both after the first postoperative year and at two years of age. The very early group demonstrated the most rapid growth in receptive language starting from 3 months postoperation and matched the language development of their normal-hearing peers by 9 months post-operation. By age 2, they were the only group to achieve receptive language milestones comparable to those of normal-hearing children. Additionally, our analysis indicated that receiving a CI before 9 months is associated with better receptive language outcomes up to 1 year postoperation and at 2 years of age, while the benefits to expressive language were less pronounced. This underscores the benefits of implantation before 9 months for children with profound bilateral deafness and a known etiology. Correlation analysis between the SELSI receptive/expressive scores and the age at CI, ranging from as early as 5 months to at least 24 months, revealed consistent negative correlations at multiple postoperative benchmarks, particularly at 6 and 9 months post-surgery. Notably, at the age of 24 months, there was a significant inverse relationship between younger ages at CI and higher receptive language scores, a pattern not observed in the expressive scores. The data clearly underscore the critical importance of performing CI before the age of 9 months in children with profound bilateral deafness and a known favorable etiology. It specifically demonstrates that the advantages of CI performed at ages younger than 9 months persist up to at least the age of 2. Furthermore, very early CI has a more significant impact on the development of receptive language skills than expressive language skills during this period.
Consistent with our findings, Culbertson et al. [10] demonstrated that implantees under 9 months of age rapidly improve in auditory skills postoperatively. By the age of 2 years, these children matched the auditory skills of their normal-hearing peers and continued to outperform those implanted later, at least until the age of 4 [10]. Similarly, Karltorp et al. [5] found that children implanted before 9 months exhibited language comprehension growth rates comparable to those of normal-hearing children, as measured by the Reynell scales, particularly up to the age of 4. It appears that initiating CI before 9 months positively impacts receptive language development (as per our study), auditory skills, and language comprehension, up until the ages of 2, 4, and 4 years, respectively. Since auditory skills precede spoken language development and predict speech production abilities [33], it is reasonable to expect that expressive language improvements, benefiting from very early CI, may emerge later than receptive language gains and may not be fully evident by age 4. Of course, we cannot rule out the possibility that after the age of 4, children in both the early and delayed CI groups might catch up to the very early group, potentially leading to no significant differences in higher language skills in the long term. Indeed, studies such as that of Dunn et al. [34] have analyzed long-term outcomes in children who received CIs before the age of 4 years, categorized by whether they were implanted before or after 2 years of age. They observed that the differences in long-term language outcomes between these groups diminished over time, suggesting that the influence of CI timing on language development decreases as children grow older [34]. However, it remains uncertain whether this trend applies to CIs performed much earlier than 2 years of age. To clarify this, longer-term studies with larger cohorts, including a significant number of very early CI and early CI groups, are necessary in future research.
Indeed, recent studies have increasingly focused on the long-term impact of CI before 9 months of age on language development. Chweya et al. [3] in the United States found that children who received CIs before 9 months of age exhibited superior receptive and expressive language development compared to those who were implanted between 9 and 12 months of age, after an average follow-up of 7.4 years. They observed a negative correlation between the age at implantation (ranging from 5 to 35 months) and language scores at the most recent follow-up, as evaluated by the Pre-school Language Scales Fourth and Fifth Editions (PLS-4 and PLS-5) and the Oral and Written Language Scales tests. This indicates that earlier CI (before 9 months) has a positive effect on long-term receptive and expressive language outcomes. Dettman et al. [9] also reported better receptive language outcomes, as measured by the Categories of Linguistic Performance, in children who received their implants very early, at age 5. Karltorp et al. [5] found that the correlation between age at CI and post-CI receptive and expressive vocabulary development did not become evident until the children were older, specifically at ages 6 and 8, respectively. Furthermore, the correlation with expressive language development appeared even later, at the age of 8, compared to the correlation with receptive language development. These findings are consistent with those from our study, which had a shorter follow-up period and did not yet show differences in expressive language development between the very early (before 9 months) and early (between 9 and 12 months) CI groups.
One intriguing point in this study is the comparison between the very early CI group and the delayed CI group. Examination of their pre-surgical hearing status revealed that the delayed CI group had significantly better preoperative speech development. Additionally, this group demonstrated a statistically significant better auditory threshold before CI, at least in one ear (Table 2, Supplementary Table 2). This finding helps explain why their CI was postponed. It is important to note that half of the individuals in the delayed CI group were diagnosed with enlarged vestibular aqueductŌĆöa condition that is historically linked to a favorable prognosis after CI [35]. However, longitudinal observations over the first postoperative year indicate that the very early CI group, though not statistically significant, tends to show higher receptive language development percentiles immediately following CI compared to the delayed CI group. By 24 months of age, the very early CI group reaches the median milestone for receptive language development, whereas the delayed CI group, despite better pre-surgery language and hearing status, does not. This pattern was not observed in the early CI group. This suggests that even when one ear has a hearing threshold between 80 and 90 dB (which is better than a profound degree), it may be wise to consider CI, at least unilaterally, before the age of 9 months to optimize receptive language outcomes. To incorporate this approach into clinical practice in South Korea, changes to the governmentŌĆÖs pediatric CI national insurance criteria are required. Currently, the criteria stipulate a hearing threshold greater than 90 dB bilaterally for children under 12 months of age.
The strengths of this study include the establishment of clear, objective criteria for CI timing in infants, with decisions made solely based on these criteria. This approach minimizes the influence of parental SES and willingness on rehabilitation outcomes. All surgeries were performed by the same surgeon at a single center, and the same type of electrodes was used in most cases, which reduces bias and variables other than the age at CI. Cases with CND, which are known for less favorable outcomes, were excluded from the performance analysis. However, one limitation of this study is that it only compares outcomes up to the age of 2 years. This necessitates long-term follow-up to determine whether the superior effects of very early CI (under 9 months) over early CI (9 to 12 months) are sustained over time. Additionally, we compared the language development scores of all children in groups A, B, and C. Due to the small number of subjects, particularly in group C, we were unable to perform a comparison of language development scores among subjects who underwent simultaneous CI. While this may be considered a potential limitation of the study, the majority of subjects in all groups received simultaneous implants. Therefore, we do not anticipate a significant difference in language development outcomes when comparing only those who underwent simultaneous CI. For those who did not receive simultaneous implants but underwent sequential or unilateral CI, we believe that their inclusion in the comparison did not bias the results in favor of group A. This is because individuals, especially in groups B or C, likely had more residual hearing or more advanced language development prior to receiving their CI.
In conclusion, we established criteria for determining the appropriate timing of CI in infants, which are based on objective assessments of hearing and language development status. These criteria are independent of the parentsŌĆÖ financial capacity or willingness to pursue rehabilitation, thanks to the provision of government insurance coverage. According to our findings, which are the first of their kind to our knowledge, infants who receive off-label, very early CI (under 9 months of age) demonstrate more advanced receptive and expressive language development in the first year post-implantation than those who receive early CI (between 9 and 12 months of age). Although the gains in expressive language are less pronounced during this period, they are still noteworthy. Remarkably, only the very early CI group (under 9 months of age) shows significant improvement in receptive language beginning at 3 months post-CI. These infants rapidly reach the level of their normal-hearing peers by 9 months postoperatively and maintain this advanced level at least until the age of two. It is also significant that the very early CI group tends to exhibit superior receptive language development during this period compared to the delayed CI group, where implantation is postponed until after 12 months of age due to the presence of significant residual hearing and advanced preoperative language development. These findings support the consideration of earlier CI in infants with documented etiologies and speech delays, without being restricted to the pediatric FDA labeling criteria, which currently recommend implantation at 9 months of age or older.
Ō¢¬ This study examined a unique cohort where the etiology of hearing loss was identified through genetic or radiological testing in over 90% of pediatric implantees.
Ō¢¬ The study highlights the benefits of very early cochlear implantation (CI) in infants, with a focus on those implanted before 9 months, showing superior receptive language development compared to those implanted later.
Ō¢¬ Only the very early CI group (<9 months) demonstrated notable improvement in receptive language starting at 3 months post-CI, rapidly reaching the level of normal-hearing peers by postoperative 9 months, and impressively maintained this advanced level at least until the age of 2 years.
Ō¢¬ The studyŌĆÖs strength lies in its objective criteria for the choice of CI timing, minimizing parental socioeconomic influences, although longer-term follow-up is needed to confirm the long-term benefits of CI under 9 months of age.
CONFLICT OF INTERESTByung Yoon Choi is an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported. NotesAUTHOR CONTRIBUTIONS Conceptualization: SJL, BJK, BYC. Data curation: SJL, HO, KHS, SMP, YKK, DHJ, JY, YC. Formal analysis: SJL. Funding acquisition: SJL, BYC. Investigation: JY, YC, MYK, JHH, JAK, NTT. Methodology: SJL, BYC. Supervision: BJK, BYC. Validation: SJL, BJK, BYC. Visualization: SJL. WritingŌĆōoriginal draft: SJL. WritingŌĆōreview & editing: BJK, BYC. ACKNOWLEDGMENTSThis study was supported by a 2023 Inje University research grant (granted to SJL), by the Basic Science Research Program through the NRF, funded by the Ministry of Education (Grant 2021R1A2C2092038 to BYC), the Bio Core Facility center program through the NRF-2022M3A9G1014007 (granted to BYC), the Basic Research Laboratory program through the NRF, funded by the Ministry of Education (Grant RS-2023-0021971031 482092640001 to BYC) and the Technology Innovation Program (K_G012002572001 to BYC) funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea). This study was also funded by SNUBH intramural research fund (13-2022-0010, 02-2017-0060, and 13-2017-0013 to BYC).
SUPPLEMENTARY MATERIALSSupplementary materials can be found online at https://doi.org/10.21053/ceo.2024.00011.
Supplementary┬ĀTable┬Ā1.Speech delay in months equivalent to the bottom one percentile (<1%) of their normal-hearing peers of the same age according to the SELSI standards Supplementary┬ĀTable┬Ā2.Preoperative audiological results within the non-CND group Supplementary┬ĀTable┬Ā3.Mean percentile results of SELSI receptive and expressive scores within pediatric patients diagnosed with CND Supplementary┬ĀFig.┬Ā1.Mean percentile results of Sequenced Language Scale for Infants receptive and expressive scores in pediatric patients diagnosed with cochlear nerve deficiency (CND). The CND group showed significantly poor outcomes at most postoperative time points in both receptive and expressive scores. Postop, postoperative. Fig.┬Ā1.The overall number of patients at each age at implantation. The average age at cochlear implantation (CI) of our total pediatric cohort was 12.5┬▒7.1 months. They were divided into three subgroups: < 9 months (very early CI group), 9ŌĆō12 months (early CI group), and 12ŌĆō18 months group (delayed CI group). 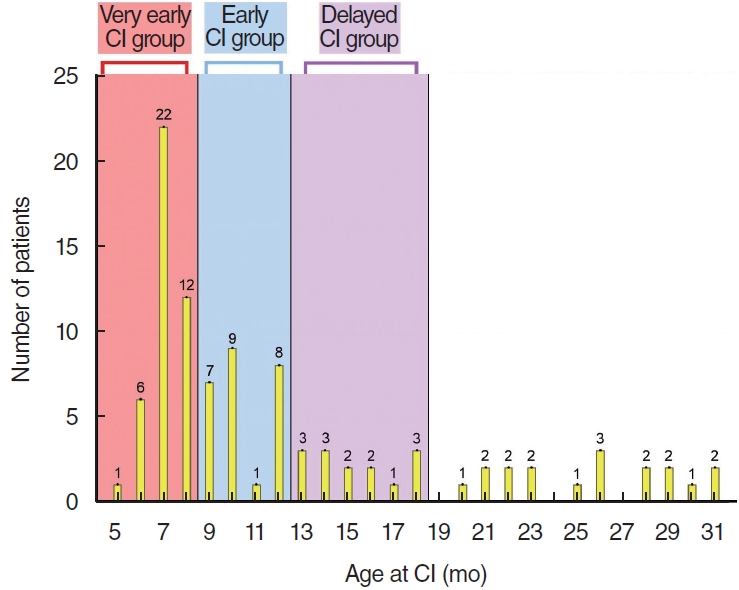
Fig.┬Ā2.Correlation analysis between Sequenced Language Scale for Infants (SELSI) receptive (A) and expressive (B) scores and age at implantation within the non-cochlear nerve deficiency patients demonstrated overall negative correlations at all postoperative time points. (C) Receptive scores at 24 months of age also indicated significantly negative correlations with younger ages at the time of implantation, while expressive scores did not. Postop, postoperative; CI, cochlear implant. 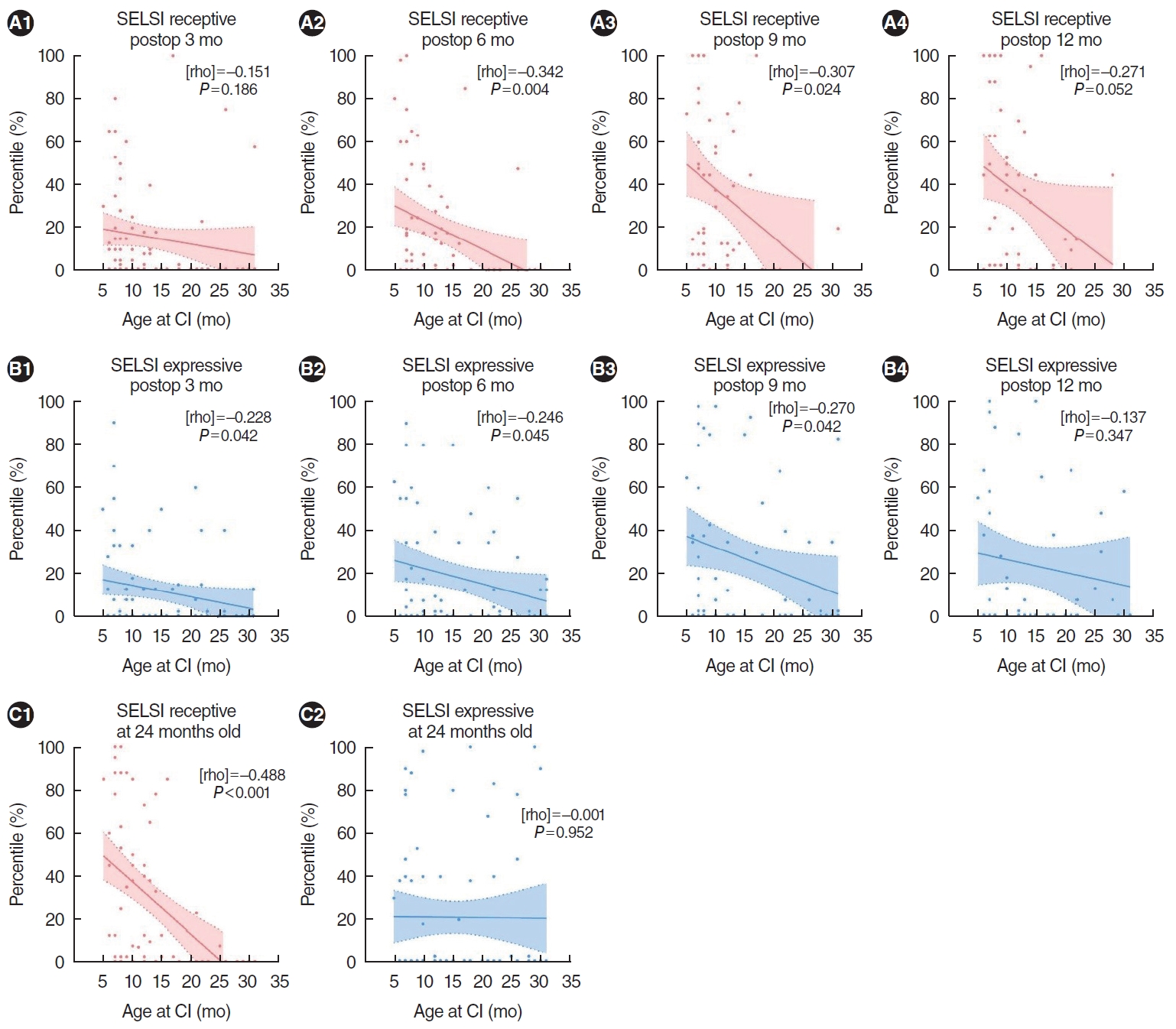
Table┬Ā1.A comparison of baseline characteristics by age groups Table┬Ā2.Mean percentile SELSI receptive and expressive scores of all non-CND patients between the three subgroups
REFERENCES1. Food and Drug Administration. Summary of safety and effectiveness data (SSED): cochlear implant (CI) system [Internet]. Food and Drug Administration; 2020 [cited 2024 Feb 2]. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf/P970051S172B.pdf.
2. Food and Drug Administration. Drug safety communication: FDA approves label changes for use of general anesthetic and sedation drugs in young children [Internet]. Food and Drug Administration; 2017 [cited 2024 Feb 2]. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-approves-label-changes-use-general-anesthetic-and-sedation-drugs.
3. Chweya CM, May MM, DeJong MD, Baas BS, Lohse CM, Driscoll CL, et al. Language and audiological outcomes among infants implanted before 9 and 12 months of age versus older children: a continuum of benefit associated with cochlear implantation at successively younger ages. Otol Neurotol. 2021 Jun;42(5):686-93.
4. Leigh J, Dettman S, Dowell R, Briggs R. Communication development in children who receive a cochlear implant by 12 months of age. Otol Neurotol. 2013 Apr;34(3):443-50.
5. Karltorp E, Eklof M, Ostlund E, Asp F, Tideholm B, Lofkvist U. Cochlear implants before 9 months of age led to more natural spoken language development without increased surgical risks. Acta Paediatr. 2020 Feb;109(2):332-41.
6. Wie OB. Language development in children after receiving bilateral cochlear implants between 5 and 18 months. Int J Pediatr Otorhinolaryngol. 2010 Nov;74(11):1258-66.
7. Naik AN, Varadarajan VV, Malhotra PS. Early pediatric cochlear implantation: an update. Laryngoscope Investig Otolaryngol. 2021 May;6(3):512-21.
8. Chweya CM, Smith AJ, May MM, Lohse CM, Neff BA, Driscoll CL, et al. Prevalence of surgical, anesthetic, and device-related complications among infants implanted before 9 and 12 months of age versus older children: evidence for the continued expansion of pediatric cochlear implant candidacy criteria. Otol Neurotol. 2021 Jul;42(6):e666-74.
9. Dettman S, Choo D, Au A, Luu A, Dowell R. Speech perception and language outcomes for infants receiving cochlear implants before or after 9 months of age: use of category-based aggregation of data in an unselected pediatric cohort. J Speech Lang Hear Res. 2021 Mar;64(3):1023-39.
10. Culbertson SR, Dillon MT, Richter ME, Brown KD, Anderson MR, Hancock SL, et al. Younger age at cochlear implant activation results in improved auditory skill development for children with congenital deafness. J Speech Lang Hear Res. 2022 Sep;65(9):3539-47.
11. Olsen LB, Larsen S, Wanscher JH, Faber CE, Jeppesen J. Postoperative infections following cochlear implant surgery. Acta Otolaryngol. 2018 Oct;138(10):956-60.
12. Byun H, Moon IJ, Kim EY, Park J, Kwon SY, Han HD, et al. Performance after timely cochlear implantation in prelingually deaf children with cerebral palsy. Int J Pediatr Otorhinolaryngol. 2013 Jun;77(6):1013-8.
13. Caragli V, Monzani D, Genovese E, Palma S, Persico AM. Cochlear implantation in children with additional disabilities: a systematic review. Children (Basel). 2023 Oct;10(10):1653.
14. Suh MJ, Lee HJ, Choi HS. Early linguistic developments of simultaneous bilateral cochlear implantees. Korean J Otorhinolaryngol Head Neck Surg. 2018 Dec;61(12):650-7.
15. Balasundaram P, Avulakunta ID. Bayley scales of infant and toddler development [Internet]. StatPearls Publishing; 2022 [cited 2024 Feb 2]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK567715/.
16. Kim YT. Content and reliability analyses of the Sequenced Language Scale for Infants (SELSI). Commun Sci Disord. 2002;7(2):1-23.
17. Kim YT, Hong GH, Kim KH. Content and reliability analyses of the receptive and expressive vocabulary test (REVT). Commun Sci Disord. 2009;14(1):34-45.
18. Moon IJ, Kim EY, Chu H, Chung WH, Cho YS, Hong SH. A new measurement tool for speech development based on LingŌĆÖs stages of speech acquisition in pediatric cochlear implant recipients. Int J Pediatr Otorhinolaryngol. 2011 Apr;75(4):495-9.
19. Han MJ, Min JH, Kim SJ. Effect of oxcarbazepine on language function in patients with newly diagnosed pediatric epilepsy. J Clin Neurol. 2023 Jan;19(1):76-82.
20. Yoon JA, An SW, Choi YS, Seo JS, Yoon SJ, Kim SY, et al. Correlation of language assessment batteries of toddlers with developmental language delay. Ann Rehabil Med. 2022 Oct;46(5):256-62.
21. Min BJ, Kim N, Chung T, Kim OH, Nishimura G, Chung CY, et al. Whole-exome sequencing identifies mutations of KIF22 in spondyloepimetaphyseal dysplasia with joint laxity, leptodactylic type. Am J Hum Genet. 2011 Dec;89(6):760-6.
22. Han KH, Kim AR, Kim MY, Ahn S, Oh SH, Song JH, et al. Establishment of a flexible real-time polymerase chain reaction-based platform for detecting prevalent deafness mutations associated with variable degree of sensorineural hearing loss in Koreans. PLoS One. 2016 Sep;11(9):e0161756.
23. Lee SY, Oh DY, Han JH, Kim MY, Kim B, Kim BJ, et al. Flexible realtime polymerase chain reaction-based platforms for detecting deafness mutations in Koreans: a proposed guideline for the etiologic diagnosis of auditory neuropathy spectrum disorder. Diagnostics (Basel). 2020 Sep;10(9):672.
24. Kim BJ, Kim AR, Lee C, Kim SY, Kim NK, Chang MY, et al. Discovery of CDH23 as a significant contributor to progressive postlingual sensorineural hearing loss in Koreans. PLoS One. 2016 Oct;11(10):e0165680.
25. Kim Y, Han JH, Yoo HS, Choi BY. Molecular aetiology of ski-slope hearing loss and audiological course of cochlear implantees. Eur Arch Otorhinolaryngol. 2022 Oct;279(10):4871-82.
26. Choi BY, Park G, Gim J, Kim AR, Kim BJ, Kim HS, et al. Diagnostic application of targeted resequencing for familial nonsyndromic hearing loss. PLoS One. 2013 Aug;8(8):e68692.
27. Kim NK, Kim AR, Park KT, Kim SY, Kim MY, Nam JY, et al. Whole-exome sequencing reveals diverse modes of inheritance in sporadic mild to moderate sensorineural hearing loss in a pediatric population. Genet Med. 2015 Nov;17(11):901-11.
28. Deep NL, Purcell PL, Gordon KA, Papsin BC, Roland JT Jr, Waltzman SB. Cochlear implantation in infants: evidence of safety. Trends Hear. 2021 Jan-Dec;25:23312165211014695.
29. Hoff S, Ryan M, Thomas D, Tournis E, Kenny H, Hajduk J, et al. Safety and effectiveness of cochlear implantation of young children, including those with complicating conditions. Otol Neurotol. 2019 Apr;40(4):454-63.
30. Miyamoto RT, Colson B, Henning S, Pisoni D. Cochlear implantation in infants below 12 months of age. World J Otorhinolaryngol Head Neck Surg. 2018 Feb;3(4):214-8.
31. Roland JT Jr, Cosetti M, Wang KH, Immerman S, Waltzman SB. Cochlear implantation in the very young child: long-term safety and efficacy. Laryngoscope. 2009 Nov;119(11):2205-10.
32. Dettman S, Choo D, Dowell R. Barriers to early cochlear implantation. Int J Audiol. 2016;55 Suppl 2:S64-76.
33. Tait ME, Nikolopoulos TP, Lutman ME. Age at implantation and development of vocal and auditory preverbal skills in implanted deaf children. Int J Pediatr Otorhinolaryngol. 2007 Apr;71(4):603-10.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||







