A Polymorphism (rs1801018, Thr7Thr) of BCL2 is Associated with Papillary Thyroid Cancer in Korean Population
Article information
Abstract
Objectives
Among the apoptosis signals, B-cell CLL/lymphoma 2 (BCL2) is a well-known regulator of apoptosis with anti-apoptotic properties. We investigated here whether single nucleotide polymorphisms (SNPs) of the BCL2 were associated with host susceptibility of papillary thyroid cancer (PTC) occurrence and clinicopathologic parameters.
Methods
Ninety-two PTC patients and 222 control subjects were recruited. One promoter SNP (rs2279115, -938A/C) and one synonymous SNP (rs1801018, Thr7Thr) in the BCL2 gene were selected and genotyped using direct sequencing. Multiple logistic regression models were performed to evaluate odds ratios, 95% confidence intervals, and P-values.
Results
rs1801018 of the BCL2 gene was not associated with the development of PTC. In the clinicopathologic features, rs1801018 SNP was associated with the number and location. The G allele frequency of rs1801018 in PTC patients with multifocality (13.3%) was about four-fold higher than that in PTC patients with unifocality (3.4%). The G allele frequency of rs1801018 in PTC patients with both lobes (15.4%) was increased by about five-fold, compared to PTC patients with one lobe (3.2%).
Conclusion
The results suggest that synonymous SNP rs1801018 and the G allele of the BCL2 gene may be associated with the multifocality and bilaterality of PTC in Korean population.
INTRODUCTION
Thyroid cancer is the most common cancer between ages 15 years and 64 years, and the most common cancer in women in Korea (1). The incidence of thyroid cancer has increased during the past 15-20 years, particularly for papillary thyroid cancer (PTC), which is the predominant subtype of malignant thyroid cancers (2, 3). Ionizing radiation exposure, diet, smoking, and hormonal factors are the risk factors for thyroid cancer (4, 5). Genetic predisposition has been implicated as a risk factor for PTC (6, 7). Case-control studies based on large populations have demonstrated that the genetic risk of thyroid cancer is about three to eight fold higher than that of any other major cancer (8, 9).
Apoptosis is involved in the destructive action of autoimmune thyroid disorders and in cell death of thyroid cancer. Apoptosis, programmed cell death, plays a crucial role in the prevention of uncontrolled cell growth and cancerous cells have developed mechanisms to avoid apoptosis. B-cell CLL/lymphoma 2 (BCL2) is known to protect cells from apoptosis and is firstly found in the BCL2 family. Members of BCL2 family can be classified into anti-apoptotic and pro-apoptotic proteins. Anti-apoptotic BCL2 family members are BCL2, BCL2-like 1 (BCL2L1, transcript BCLXL), BCL2L2, and myeloid cell leukemia sequence 1 (BCL2-related) (MCL1, transcript MCL1L). Pro-apoptotic BCL2 family members are divided into two types according to BCL2 homolog (BH) domains (BH1-4). Major pro-apoptotic BCL2 family members containing only BH3 domain are BCL2-associated agonist of cell death (BAD), BH3 interacting domain death agonist (BID), BCL2-like 11 (apoptosis facilitator) (BCL2L11), Bcl2 modifying factor (BMF), and MCL1 (transcript MCL1S). Minor members containing other domains include BCL2-associated X protein (BAX), BCL2-related ovarian killer (BOK), BCL2-antagonist/killer 1 (BAK1), and BCL2L1 (transcript BCLXS) (10, 11). The BCL family has an important role in the pathogenesis of thyroid cancer (12-14). In PTC, the apoptotic index correlates inversely with BCL2 expression (13). BCL2 expression is associated with differentiation of thyroid cancer (12), but a study found no relationship between BCL2 expression and the thyroid cancer differentiation (14). The role of BCL2 on the development and clinicopathologic features of thyroid cancer is largely unknown. Single nucleotide polymorphisms (SNPs) of BCL2 have been studied in several cancers, including chronic lymphocytic leukemia (15, 16), prostate cancer (17), ovarian cancer (18), breast cancer (19), renal cancer (20), oropharyngeal squamous cell carcinoma (21), and sporadic medullary thyroid carcinoma (22). To date, no study has investigated for association between BCL2 SNPs and PTC. The aim of this study was to investigate whether promoter SNP (rs2279115, -938A/C) and synonymous SNP (rs1801018, Thr7Thr) in the BCL2 gene were associated with PTC development in the Korean population. We also investigated for relationships between BCL2 SNPs and cliniopathologic features, such as size, number (unifocality and multifocality), location (one lobe and both lobe), extrathyroid invasion, and lymph node metastasis.
MATERIALS AND METHODS
Patients and controls
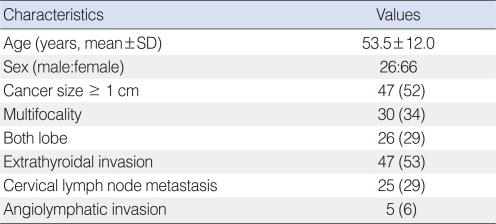
PTC patients were enrolled at the Kyung Hee University Medical Center, Seoul, Korea between October 2007 and December 2010. Control subjects were selected from healthy individuals examined under a general health check-up program without clinical evidence of cancers, thyroid diseases, or any other severe conditions. PTC and the cervical regional lymph node metastasis were confirmed by pathologic examination. Specimens confirmed as benign tumor, follicular variant, diffuse sclerosing and tall cell variants were excluded. Fifteen patients of 170 were excluded. The patient group (n=92) was comprised of 26 males and 66 females. The mean age of the patients was 53.5±12.0 years (mean±standard deviation [SD]). The control group was consisted of 222 healthy adults (53.8±6.0 years), comprised of 94 males and 128 females. This study was approved by the ethics review board of the Medical Research Institute, Kyung Hee University Medical Center (KMC IRB 1010-05). Written informed consent was obtained from each subject prior to study entry.
Patient subgroups
To determine the nature of the relationship between BCL2 SNPs and the clinicopathologic characteristics of PTC, patients were divided into subgroups according to tumor size (<1 cm and ≥1 cm), number of tumors (unifocality and multifocality), location of cancer (one lobe and both lobe), extrathyroid invasion (presence and absence), angiolymphatic invasion (presence and absence) and cervical lymph node metastasis (presence and absence). Demographic characteristics of PTC patients are summarized in Table 1; small differences in subgroup numbers were caused by loss of clinical data.
SNP selection and genotyping
We searched the promoter and coding SNPs of the BCL2 gene in the SNP database of the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/SNP, BUILD 132). SNPs with unknown heterozygosity, minor allele frequency (MAF) below 10%, and unknown genotype in Asians were excluded. Out of 7 SNPs in the promoter region, there were 3 SNPs with no heterozygosity data, 1 SNP with heterozygosity below 0.05, and 2 SNPs with unknown genotype in Asians. Among 5 coding SNPs, 3 SNPs with heterozygosity were below 0.05 and 1 SNP was with unknown MAF. Finally, two SNPs (rs2279115, -938A/C; rs1801018, Thr7Thr) were selected. These SNPs were also cited in several studies. Blood samples for DNA extraction from each subject were collected in EDTA tube and then stored in a -80℃ refrigerator. Genomic DNA was extracted using a QIAamp® DNA mini kit (QIAGEN, Valencia, CA, USA). SNP genotyping was determined by direct sequencing. Polymerase chain reactions (PCRs) were performed using specific primers for BCL2 SNPs that were selected for analysis: for rs2279115 (sense, 5'-ATAAAACCCTCCCCCACCACCT-3'; antisense, 5'-ACAATTTTCAGTCCGGTATTCG-3'; product size, 343 bp) and for rs1801018 (sense, 5'-GGGGAAACACCAGAATCAAGT-3'; antisense, 5'-GGGCTGGGAGGAGAAGATGC-3'; product size, 334 bp). The PCR products were sequenced using an ABI PRISM 3730XL analyzer (PE Applied Biosystems, Foster City, CA, USA). Sequencing data were analyzed using SeqManII software (DNASTAR, Madison, WI, USA).
Statistical analysis
Continuous variables are presented as mean±SD using the independent t-test and chi square test. Hardy-Weinberg equilibrium (HWE) was calculated using the SNPStats software (http://bioinfo.iconcologia.net/index.php?module=Snpstats) in both patient and control groups. SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA) program and SNPStats were used to analyze genetic data. Linkage disequilibrium (LD) block and haplotypes were evaluated using Haploview version 4.2 (23). Multiple logistic regression models adjusted for gender and sex (codominant1, codominant2, dominant, recessive, overdominant, and log-additive) were employed to obtain odds ratios (ORs), 95% confidence intervals (CIs), and P-values. Fisher exact test and Bonferroni correction were also performed. Statistical significance was set at P<0.05.
RESULTS
Genotypic distributions of the two SNPs examined in this study were in HWE (P>0.05, data not shown). Genetic associations between BCL2 and PTC were investigated. Multiple logistic regression analysis was performed: for the promoter SNP rs2279115, -938A/C (codominant 1, C/C vs. A/C; codominant 2, C/C vs. A/A; dominant, C/C vs. A/C + A/A; recessive, C/C + A/C vs. A/A; overdominant, C/C + A/A vs. A/C; log-additive, C/C vs. A/C vs. A/A) and for the synonymous SNP rs1801018, Thr7Thr (codominant 1, A/A vs. A/G; codominant 2, A/A vs. G/G; dominant, A/A vs. A/G + G/G; recessive, A/A + A/G vs. G/G; overdominant, A/A + G/G vs. A/G; log-additive, A/A vs. A/G vs. G/G).
In analyses of genotype data from 92 PTC patients and 222 controls, the frequencies of BCL2 SNP (rs1801018, Thr7Thr) were different in PTC and control (dominant model [A/A+A/G vs. G/G]: OR, 0.51; 95% CI, 0.26 to 1.00; P=0.042; overdominant model [G/G vs. A/A]: OR, 0.49; 95% CI, 0.25 to 0.99; P=0.036). However, the association disappeared after performing Bonferroni correction. The BCL2 promoter SNP (rs2279115, -938A/C) was not associated with PTC (Table 2).
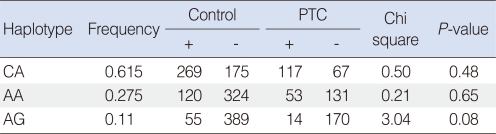
One LD block by the Gabriel method was made (24). LD block was consisted of rs2279115 and rs1801018 of BCL2, and 3 haplotypes were constructed (Fig. 1). In haplotype analysis, haplotypes were not associated with PTC (Table 3).
When we assessed the genetic relationships between SNPs and subgroups of PTC patients, BCL2 SNP (rs1801018) was significantly associated with PTC multifocality (P=0.036 in genotype frequency after Bonferroni correction; P=0.046 in allele frequency after Bonferroni correction) and bilaterality (P=0.008 in genotype frequency after Bonferroni correction; P=0.012 in allele frequency after Bonferroni correction) (Tables 4 and 5). The G allele frequencies of rs1801018 in PTC patients with multifocality (13.3%) were about four-fold higher than those in PTC patients with unifocality (3.4%). The G allele frequencies of rs1801018 in PTC patients with both lobes (15.4%) were increased by about five-fold compared to PTC patients with one cancerous lobe (3.2%). However, BCL2 SNPs (rs2279115 and rs1801018) were not associated with size, extrathyroid invasion, angiolymphatic invasion or lymph node metastasis.

Genotype and allele frequencies of SNPs of BCL2 gene in PTC patients with unifocality and PTC patients with multifocality
DISCUSSION
This is the first study to investigate the association between BCL2 SNPs and PTC in the Korean population. We analyzed the promoter SNP (rs2279115, -938A/C) and synonymous SNP (rs1801018, Thr7Thr) in PTC patients and control subjects. We also assessed the relationships between two SNPs (rs2279115, -938A/C; rs1801018, Thr7Thr) and the clinicopathologic characteristics of PTC. Our results showed that synonymous SNP (rs1801018, Thr7Thr) of the BCL2 was weakly associated with the development of PTC (P=0.042 in dominant model, P=0.082 after Bonferroni correction; P=0.036 in dominant model, P=0.072 after Bonferroni correction). Haplotype was not different between PTC patients and control subjects. Regarding the clinicopathologic features of PTC, the synonymous rs1801018 SNP was associated with the number (unifocality and multifocality) (P=0.018 in genotype frequency adjusting Fisher exact test, P=0.036 after Bonferroni correction; P=0.023 in allele frequency adjusting Fisher exact test, P=0.046 after Bonferroni correction) and the location (one lobe and both lobe) (P=0.004 in genotype frequency adjusting Fisher exact test, P=0.008 after Bonferroni correction; P=0.006 in allele frequency adjusting Fisher exact test, P=0.012 after Bonferroni correction).
In recent times, several genetic studies have reported relationship between PTC and candidate genes, such as WD repeat domain 3 (WDR3) (25), vascular endothelial growth factor A (VEGFA) (26), RAD52 homolog (S. cerevisiae) (RAD52) (27), protein tyrosine phosphatase, receptor type, J (PTPRJ) (28), purinergic receptor P2X, ligand-gated ion channel, 7 (P2X7) (29), interleukin 6 (interferon, beta 2; IL6) (30), IL10 (31) and IL11 (32).
There is one study that investigated for the relationship between BCL2 SNPs and thyroid cancer. Ruiz-Llorente et al. (22) reported that a BCL2 SNP was associated with the development of sporadic medullary thyroid cancer (MTC) in the Spanish population. The genotype distribution of the rs1801018 SNP was different between MTC patients and controls (P=0.002 in codominant model, P=0.56 after Bonferroni correction). Their study did not show the promoter SNP (rs2279115, -938A/C) of BCL2 and the clinicopathologic characteristics, such as size, number, location, extrathyroidal invasion, and lymph node metastasis of cancers. Although we use PTC instead of MTC, the results show similar patterns. For the exact correlation between BCL2 and thyroid cancer, other studies with larger sample size and based on different populations will be needed. In this study, associations between synonymous SNP (rs1801018, Thr7Thr) and the clinicopathologic features of PTC were found. The rs1801018 was associated with susceptibility to the number of PTC. The A/G genotype and G allele frequencies of rs1801018 in PTC patients with multifocality (A/G genotype, 26.7%; G allele, 13.3%) were about four-fold higher than those in PTC patients with unifocality (A/G genotype, 6.8%; G allele, 3.4%). The rs1801018 was also associated with the location of PTC. The A/G genotype and G allele frequencies of rs1801018 in PTC patients with both lobes (A/G genotype, 30.8%; G allele, 15.4%) were increased by about five-fold, compared to PTC patients with one lobe involvement (A/G genotype, 6.3%; G allele, 3.2%). Therefore, we propose that rs1801018 could be a useful marker for the number and location of PTC, and the G allele of rs1801018 may be a risk factor for multifocality or bilaterality of PTC. Although PTC has a good prognosis following appropriate treatment, the multifocality and bilaterality of PTC are indications for complete thyroidectomy and post-operative radioactive iodine ablation.
To determine whether the promoter SNP affects transcription factors, we used the online program AliBaba 2.1 (http://www.gene-regulation.com/pub/programs/alibaba2). At the sites of the rs2279115 SNP in BCL2, the A-containing sequences can bind with Id2 and SP1 transcription factors, and the G-containing sequences bind only with SP1. Assuming transcription factor binding varies with promoter SNPs, this promoter SNP may influence gene and protein expressions of BCL2. This study has several limitations. The control group did not undergo thyroid ultrasonography to exclude potentially undetected thyroid cancers. Considering the incidence of thyroid cancer (0.5-10/100,000 person), this limitation imposes low impact on this study. Small sample size was another limitation. To confirm our results, additional study with larger sample size should be conducted. Another limitation is absence of study using tumor tissue, the genetic changes of BCL2 will be examined in a cell line and immunohistochemical study in specimen tissue to determine the biologic effects.
In conclusion, our results indicate that synonymous SNP rs2010963 (Thr7Thr) of the BCL2 gene may be associated with the multifocality or bilaterality of PTC, and the G allele could be a risk factor for multifocality or bilaterality of PTC in Korean population.
ACKNOWLEDGEMENT
This present study was sponsored by Hyoseok Research Fund.
Notes
No potential conflict of interest relevant to this article was reported.