Double Glomus Tumors Originating in the Submandibular and Parotid Regions
Article information
Abstract
Glomus tumors are rare neoplasms that originate from the glomus bodies, an arteriovenous anastomosis with a specialized vascular structure. The most common site for these tumors is the subungal region of the fingers. Occasionally, glomus tumors are found in the middle ear, trachea, nasal cavities, stomach, and lungs. The occurrence in the parotid regions is very rare. While multiple glomus tumors in the whole body are thought to represent only 10% of all cases, instances of multiple tumors in the neck have not yet been reported in the literature. We report a case of double glomus tumors in the submandibular and parotid regions.
INTRODUCTION
The glomus body is an apparatus located between the arterial and venous systems in the skin. It is encapsulated and works as a shunt between the arterioles and the venous blood, and is referred to as the Sucquet-Hoyer canal (1). These structures regulate skin temperature (2). Occasionally, neoplasms develop at the glomus apparatus. A glomus tumor often appears as a slow growing, small, blue-red nodule that, clinically, might be confused with a hemangioma. Glomus tumors, first described in 1924 (3), are benign neoplasms of the normal glomus bodies.
Multiple glomus tumors represent only 10% of all cases (4). Their relative rarity is associated with limited recognition of the condition. The following report describes a case of two glomus tumors originating in the submandibular area and parotid gland.
CASE REPORT
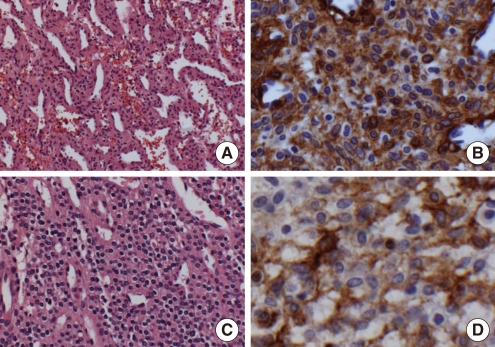
A 55-years-old man was referred to our department with a 5-month history of painful masses in the left portion of the neck. The medical history was unremarkable. Two masses were palpated in the right parotid region and submandibular (level IIA) area. Computerized tomography scans revealed a tumor in the right parotid region and another in the submandibular area, both approximately 3 cm in diameter (Fig. 1). The fine needle aspiration cytology along with clinical observation showed that the lesion was similar to a hemangioma. The patient was scheduled for surgery. A modified Blair incision was used to approach the lesion. After exposing the main trunk of the facial nerve and the peripheral branches, purple colored tumors were found at the surface of the parotid gland and submandibular area. The tumors and the surrounding tissues were removed, leaving the facial nerve intact (Fig. 2). Following the operation, the patient did not have severe bleeding or facial palsy. Histologically, the tumor contained many capillaries and a few wide vessels. Glomus cells that were positive for smooth muscle actin using an immunoperoxidase stain were scattered between these cells. S-100 protein, pancytokeratin, desmin, glial fibrillary acidic protein, and HMB-45 were negative in the glomus tumor. Staining for CD34 was positive in the vascular endothelial cells, but negative in the glomus cells. The glomus cells were arranged in organoid and trabecular patterns surrounding vascular lumens. No lymphoid cells or tissues were observed in any of the sections. A diagnosis of glomus tumors was made.

Neck CT axial images with enhancement show two mass like lesions in parotid (A) and submandibular region (B) (arrows).
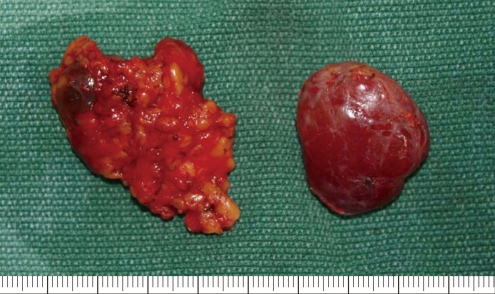
Postoperative picture show parotid region mass (left) and submandibular region mass (right) specimens.
According to the number of glomus cells, vascular structures, and smooth muscle tissue, the tumor located at the submandibular area was classified as an angiomatous type, and designated as glomangioma. The other tumor at the parotid region was classified as a solid type with masses of glomus cells, and was considered as a glomus tumor proper (Fig. 3). The postoperative period was uneventful, and no signs of recurrence have been noted.
DISCUSSION
This is the first case in the English literature showing multiple glomus tumors in the cervical region. Multiple glomus tumors are thought to represent only 10% of all cases of glomus tumors (4). The relative rarity of multiple glomus tumors and variation in presentation has limited the awareness and recognition of this condition. This leads to prolonged symptoms such as chronic pain, excessive testing, and misdiagnosis. Further complicating the diagnosis, glomus tumors do not have a pathognomic appearance on radiological studies.
According to the World Health Organization classification of head and neck tumors, three distinctive types of glomus tumor patterns are recognized: a solid type with masses of glomus cells, an angiomatous type called a glomangioma, and a solid type with myomatous spindle cell differentiation called a glomangiomyoma (5, 6).
Glomus tumors can be solitary or multiple, and some are familial. The latter type has been reported to be autosomal dominant with incomplete penetrance with variable expression (reference). Most familial tumors are glomangiomas. Glomangiomas constitute approximately 20% of all glomus tumors (5) and can be found throughout the body, although they are most commonly found on the fingertips. Glomangiomas constitute 1% to 5% of the soft tissue tumors of the hand (7); they can appear in other locations such as the bone, spine, liver, kidney, and stomach. In a survey of glomus tumors in the registry of the Armed Forces Institute of Pathology, head and neck tumors represented 40 out of 506 cases, six of which were glomangiomas (6). Glomus cell proliferation in a glomangioma can be inconspicuous; without a clearly described clinical presentation, it could be overlooked upon hematoxylin and eosin staining. Immunostaining with antismooth muscle actin, however, highlights the tumor cells and, together with negative cytokeratin staining, rules out any suspicion of cystic sialoadenoma. In contrast to solitary glomus tumors, which tend to occur in adulthood, multiple glomus tumors are thought to appear more commonly in childhood or adolescence (8), with rare onset after the age of 30 (9, 10). Review of the previously reported cases of multiple glomus tumors revealed a slight male predominance (8). Sixty percent of multiple glomus cases had a positive family history, supporting the belief that this abnormality is inherited in an autosomal dominant fashion with incomplete penetrance and variable expressivity. The gene responsible for this disorder has been localized to chromosome 1p21-22 (11). A 1995 report described only 12 cases of congenital multiple glomus tumor in the literature to that point (12). These congenital tumors are usually present from birth and tend to enlarge with body growth (13).
The present case illustrates the importance of a thorough physical examination of the head and neck when multiple glomus tumors are found. Any suspicious finding should be followed up with imaging. Complete excision with some degree of margin is thought to be curative (14, 15), although some alternative therapies, such as hypertonic saline injection (16), flashlamp tuneable dye laser (17), and schlerotherapy (18) have been suggested.
We hope that this report will alert clinicians to this diagnosis and aid in more rapid diagnosis and treatment.
Notes
No potential conflict of interest relevant to this article was reported.