Are Systemic Voriconazole and Caspofungin Ototoxic? An Experimental Study with Rats
Article information
Abstract
Objectives
To determine whether systemic administration of voriconazole and caspofungin causes ototoxicity.
Methods
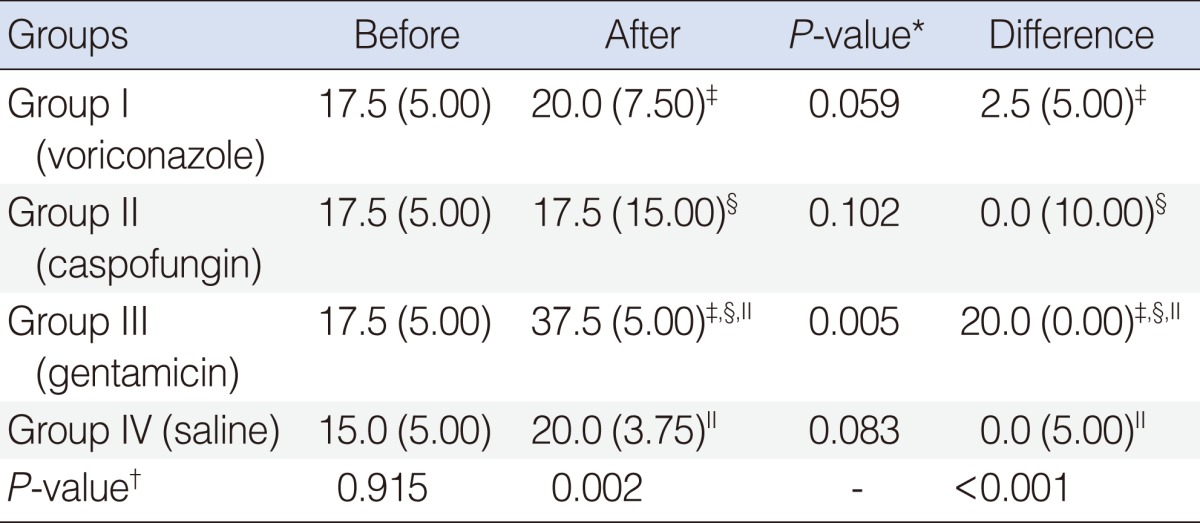
This study was conducted on 32 healthy male Wistar albino rats. The baseline auditory brainstem response (ABR) thresholds of all animals were obtained under general anesthesia. Then, the rats were randomly divided into 4 groups (groups I-IV), each group consisting of 8 rats. Rats in group I were injected intraperitoneally with voriconazole 10 mg/kg/day for 7 days, and the rats in the group II were injected intraperitoneally with caspofungin 5 mg/kg/day for 7 days. Group III received 120 mg/kg/day gentamicin for 7 days. Group IV received saline for 7 days. The animals were then observed for 7 days, and on 14th day of the trial, posttreatment ABRs of both ears were recorded.
Results
We did not find any significant differences between pretreatment and posttreatment median ABR thresholds in the voriconazole, caspofungin, or saline groups. In the gentamicin group, there was a statistically significant difference between pretreatment and posttreatment ABR thresholds.
Conclusion
Caspofungin and voriconazole did not change ABR thresholds in speech frequencies after a 7-day-period of their administration. We believe that further animal studies must be performed after administration of these agents for a longer time period, and these findings must be consolidated with histopathological investigations.
INTRODUCTION
Voriconazole and caspofungin, 2 new broad-spectrum systemic antifungal agents, have been recently preferred in the treatment of invasive fungal infections, and especially in those that arise due to Aspergillus and Candida spp [1-3]. These agents have milder side-effects despite their higher efficacy [2]. Fatal acute invasive fungal infections can develop in immunocompromised patients with bone marrow transplantation, leukemia, cancer, AIDS, diabetes mellitus, or in patients that require immunosuppressive drugs or who undergo kidney dialysis; in these cases, a systemic antifungal therapy may be required [4]. Fungal sinusitis and invasive fungal external otitis are well-known fungal infections of the head and neck region [5, 6].
Voriconazole, a synthetic derivative of fluconazole, is a second generation triazole with a broad spectrum of antifungal activity against Candida, Aspergillus, Cryptococcus, and other species [2, 3, 5]. It has a low molecular weight and very good penetration of bone and soft tissues [5, 7]. This property makes voriconazole essential for the treatment of patients with fungal mastoiditis or otogenic meningitis [5].
Caspofungin is an echinocandin antifungal agent that inhibits (1-3)-D glucan synthase activity in the fungal wall, and it is active against Aspergillus and Candida infections [4, 8]. As (1,3)-D glucan is not found in mammalian cells, inhibition of the synthesis of this molecule in fungi is highly specific, hence, it causes minimal toxicity to humans [8, 9]. The drug is well tolerated and has a low incidence for adverse effects and serious drug-drug interactions [1].
Identification of potential ototoxicity might effect the choice of the most appropriate agent for treatment. It would be valuable to know the possible ototoxic effect of the drug, even under those conditions in which the treatment regimen cannot be safely altered. This information helps the physician to inform the patient about a possible hearing loss after treatment and the possibility of a requirement for hearing aids.
To our knowledge, no studies have investigated the ototoxic effects of systemic antifungal agents up to date. We have designed the current study to assess whether systemic administration of voriconazole and caspofungin cause ototoxic effects, as determined with auditory brainstem response (ABR) thresholds.
MATERIALS AND METHODS
This study was conducted in the Animal Laboratory of Ankara Training and Research Hospital after the approval of the Ethical Committee of Ankara Training and Research Hospital (No: 1/16) and it was complied with experimental ethical principles and animal protection laws according to the rules and regulations in Turkey. All animals' care and procedures were performed humanely. The animals were kept in ordinary cages at a temperature of 20℃ to 22℃ with free access to food and water, and were subjected to a 12-hour artificial light/dark cycle. They were given pellets (2,700 ME kcal/kg, 23% HP) and water ad libitum, and were used after one week of quarantine and acclimatization. Thirty two healthy male Wistar albino rats weighing between 250 g and 280 g were used in this study. None of them had external or middle ear infection or tympanic membrane adhesion, perforation or retraction under otomicroscopic examination. The animals were anesthetized with ketamine hydrocloride (50 mg/kg, intramuscular) and xylasine (5 mg/kg, intramuscular) before obtaining their baseline ABR recordings. Then, the rats were randomly divided into 4 groups (groups I-IV), each group consisting of 8 rats. In group I voriconazole 10 mg/kg/day (Vfend, Pfizer, Istanbul, Turkey), and in group II caspofungin 5 mg/kg/day (Candidas, Merck Sharp & Dohme, Istanbul, Turkey) were injected intraperitoneally (ip) for 7 days [6]. Group III served as the positive control and received gentamicin (120 mg/kg/day) ip for 7 days. Group IV served as the negative control and the animals were injected 2 mL of saline ip for 7 days. The animals were then observed for 7 days, and on 14th day of the trial, posttreatment ABRs of both ears were recorded under general anesthesia. The recordings were acquired through a single channel using Interacoustics EP25 instrument evoked potential unit (ver. 3.00, Assens, Denmark) in a quiet room. We used subdermal needle electrodes. The noninverting (active) electrode was placed at the vertex, in the midline of the scalp, and the inverting (reference) electrode was placed in the mastoid area of tested ear. The ground electrode was inserted in the back of the rat. We used a E-A-RTONE 3A insert earphone. The ABR test was performed by 2,000 click stimulus within a range of 100-4,000 Hz. The stimulus rate was 30.0 pps at rarefaction polarity. The measurement was conducted by lowering the sound level by 20 dB decrements, starting from 110 dB. Repeatability was confirmed and the thresholds were determined by testing twice. ABR threshold was defined as the minimum intensity at which the Wave III could be identified. Posttreatment and pretreatment ABRs were compared.
Statistical analysis
Data analysis was performed by using SPSS ver. 11.5 (SPSS Inc., Chicago, IL, USA). Normal distribution of continuous variables was tested using Shapiro Wilk test. Data were shown as medians (interquartile range). Intra-group differences of pre- and posttreatment measurements were analyzed using Wilcoxon sign rank test while Kruskal-Wallis test was used to analyze inter-group differences. Multiple comparison test was used to identify the different group if the P-value yielded by the Kruskal-Wallis test was statistically significant. A P-value less than 0.05 was considered as statistically significant. Bonferroni correction was applied in multiple comparison tests to control type I error.
RESULTS
All rats were clinically healthy throughout the study. None of the animals showed signs of infection. Table 1 summarizes the ABR findings before and after treatment in all groups. Baseline ABRs were normal in both ears of all rats. We did not find significant differences in median pretreatment and posttreatment ABR thresholds of voriconazole, caspofungin, and saline groups (P=0.059, P=0.102, P=0.083, respectively).
In group III (gentamicin), the median threshold was 37.5 dB nHL after treatment. The difference between the pretreatment and posttreatment ABR thresholds was statistically significant in the gentamicin group (P=0.005). Examples of pretreatment and posttreatment ABR recordings in group I (voriconazole), group II (caspofungin), and group III (gentamicin) are shown in Figs. 1-3, respectively. The graphic shows before and after treatment median ABR thresholds in all groups (Fig. 4).

Graphs showing an example of auditory brainstem responses (ABRs) for click sitimulus before (A) and after (B) voriconazole administration. ABR thresholds were unchanged before and after voriconazole administration.
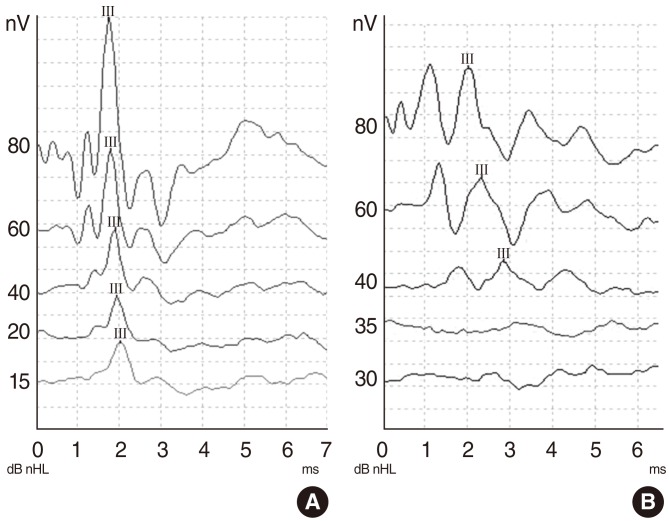
Graphs showing an example of auditory brainstem responses (ABRs) for click sitimulus before (A) and after (B) gentamicin administration. ABR thresholds before and after gentamicin administration were changed.
DISCUSSION
Ototoxic effects of some medicinal agents, including aminoglycosides, macrolides, vancomycin, loop diuretics, cisplatin, salicylate, and quinine, are well known. Although, there are limited data available on the ototoxicity of some topical antifungal agents [10], no reports have focused on the ototoxic effect of systemic antifungal agents up to date. Systemic ototoxicity may result in permanent hearing impairment and/or balance problems. It is a significant cause of vestibulocochlear morbidity [11]. Ototoxic agents may lead to degeneration of the Corti organ with loss of the external hair cells and/or damage to the inner hair cells and/or stria vascularis [10, 12].
ABR determines the velocity of nerve signal conduction along the auditory pathways starting from the cochlea up to the brainstem [12, 13]. ABR is a frequently used method in experimental animal studies to measure the alterations of the hearing thresholds [14, 15].
Despite advances in the medical practice, the incidence of invasive fungal infections, such as the infections caused by Candida and Aspergillus spp. has increased over the past 2 decades [1]. Mycotic infections of the head and neck region are uncommon and they are most frequently seen in the external auditory canal and paranasal sinuses [16]. Invasive forms of otomycosis and fungal sinusitis can develop in immunosuppressed patients, and may have lethal consequences if not treated properly [5, 17].
Fungal invasive otitis externa is an infection of the external auditory canal that involves the skull base and the mastoid cells. This invasive fungal disease may worsen progressively and may be accompanied by acute facial palsy, disequilibrium, and deafness [5]. Due to invasive nature of this life-threatening disease, prompt diagnosis and aggressive management, including surgical debridement and antifungal therapy are necessary [16]. Voricanozol has been found successful in the treatment of Aspergillus mastoiditis [16, 18].
Invasive fungal sinusitis is defined as the infiltration of mycotic organisms into the sinus mucosa. It is a rare disease in which Aspergillus species are the most frequent causative agents [2], and it is often fatal in immunocompromised patients [8]. The gold standard for treatment is wide surgical debridement, intravenous administration of antifungal agents, and correction of the underlying immunocompromised state [2]. Successful results were reported with caspofungin [8], voricanozole [2], and their combination [3, 19] in the treatment of invasive sinus aspergillosis.
Voriconazole and caspofungin are effective against molds and yeasts [20]. In an in vitro study conducted by Erbek et al. [20] it was reported that both agents showed the same activity rate (97.8%) as amphotericin B against the fungi tested. When assessed with clinical, microbiological, and histopathological techniques, caspofungin and voriconazole were shown to be as effective as amphotericin B and itraconazole, which were considered the standard regimens towards the treatment of fungal infections of the ear [6]. Voriconazole is currently considered as the first line therapeutic option for invasive aspergillosis, based on its high intrinsic anti-Aspergillus activity [7]. It has been recommended as the primary therapy for acute invasive aspergillosis in international guidelines [2]. Additionally, voriconazole has shown significant activity in patients with central nervous system and bone aspergillosis [6, 7]. Long term treatment with this antifungal agent is well tolerated and it is available for intravenous and oral use [7]. Caspofungin is a viable option for the treatment of infections due Candida spp (such as, invasive candidiasis, candidemia and esophageal candidiasis) and aspergillosis refractory to treatment [1].
To our knowledge, this is the first study that investigated the ototoxicity of systemically administered caspofungin and voriconazole. In this pilot animal study, no ototoxic effect was observed after 7 consecutive days systemic administration of caspofungin and voriconazole although they were used in higher doses than the usual daily treatment dosage. However, safety of the systemic antifungal treatment must be addressed in studies that administered these agents long term, since they must be used for a longer time in the treatment of invasive fungal disease. Short term administration of the drugs, the lack of high frequency audiometry, and histopathological examination are limitations of our study.
Caspofungin and voriconazole did not change the ABR thresholds in speech frequencies after a 7-day-period of their administration. We believe that further animal studies must be performed after administration of these agents for a longer time period, and histopathological investigations must be performed to consolidate these findings.
ACKNOWLEDGMENTS
The authors express their gratitude to Salih Ergöçen for statistical analysis, and to Cengiz Yalçınkaya, Erkan Kaya and Emir Kuşçu for their help in the animal laboratory.
Notes
No potential conflict of interests relevant to this article was reported.