Changes in the Hearing Thresholds of Infants Who Failed the Newborn Hearing Screening Test and in Infants Treated in the Neonatal Intensive Care Unit
Article information
Abstract
Objectives
The aim of this study was to investigate changes in the hearing thresholds during the first year of life in infants who failed the newborn hearing screening (NHS) test and of infants treated in the neonatal intensive care unit (NICU).
Methods
From March 2007 to November 2010, 193 healthy infants who failed the NHS test and 51 infants who were treated in the NICU were referred for evaluation of hearing acuity. Their hearing was evaluated using impedance audiometry, auditory brainstem response (ABR), and otoacoustic emission before 6 months of age, and follow-up hearing tests were administered before 12 months of age. Changes in their hearing thresholds were then analyzed.
Results
Of the 193 healthy infants who failed the NHS test, 60 infants (31%) had normal hearing acuity, 126 infants (65%) had sensorineural hearing loss (SNHL, ABR threshold ≥40 dB) and 7 infants (4%) had auditory neuropathy (AN). On the follow-up hearing tests, which were conducted in 65 infants, 6 infants showed a hearing threshold deterioration of more than 20 dB, and 19 infants showed a hearing threshold improvement of more than 20 dB. Of the 51 infants who were treated in the NICU, 38 infants (75%) had normal hearing acuity, 12 infants (24%) had SNHL, and one infant (2%) had AN. In the follow-up hearing tests, which were performed in 13 infants, one infant with normal hearing progressed to severe hearing loss. Five infants who had SNHL showed a hearing threshold improvement of more than 20 dB, and 4 infants recovered to normal hearing.
Conclusion
The hearing thresholds of infants with congenital SNHL can change during the first year of life; therefore, the importance of administration of follow-up hearing tests is emphasized. Irreversible intervention such as cochlear implantation should be considered with great caution within the first year after birth.
INTRODUCTION
Hearing loss in infancy and early childhood is not an uncommon condition. Severe bilateral sensorineural hearing loss (SNHL) affects one to three out of 1,000 live births in the healthy baby nursery population and two to four out of 100 infants in the neonatal intensive care unit (NICU) population (1). The lack of auditory input from environmental sounds and speech during early childhood due to the hearing loss interferes with the normal development of the auditory system and prevents not only the development of speech and language development but also academic achievement and social-emotional development (2, 3). Due to this concern, universal newborn hearing screening was introduced, and it has markedly improved the early detection of hearing loss in infants. An infant confirmed to have permanent SNHL should receive early intervention using an optimally fitted hearing aid. If a child with severe to profound SNHL experiences limited or no improvement from auditory habilitation using a hearing aid, cochlear implantation (CI) should be performed (4).
For the best outcome, CI should be done as early as possible to reduce the period of auditory deprivation which prohibits the normal development of the central auditory pathway. Many reports have shown clear evidence that earlier implantation results in faster development, and children implanted earlier continue to out-perform children implanted later (5, 6). Early implantation ensures that hearing-impaired children receive the maximum amount of auditory input; therefore, it reduces the adverse effects of auditory deprivation. Cochlear implantation has been approved by the US FDA in children as young as 12 months. To further reduce the period of auditory deprivation, implantation in hearing-impaired infants younger than 12 months of age has also been performed. In fact, a few reports have shown that implantation before 12 months of age provides normalization of audio-phonologic development and is associated with better speech-language development than implantation after 12 months of age (5, 6).
Precise estimation of hearing thresholds is required to select candidates for CI among infants with SNHL, and CI should be performed only after permanent severe to profound SNHL is confirmed. A caution should be employed when selecting candidates for CI among infants with SNHL because the hearing acuity of infants can change over time on rare occasions (7-9).
The aim of this study was to investigate changes in hearing thresholds during the first year of life in infants who failed the newborn hearing screening (NHS) test and in infants treated in the NICU.
MATERIALS AND METHODS
We reviewed the medical records of 244 infants who were referred to the Department of Otolaryngology at Dong-A University Hospital between March 2007 and November 2010 for evaluation of hearing acuity. We found that 193 healthy infants (108 males and 85 females) were referred for diagnostic hearing tests because they failed the NHS. Another group of 51 infants (32 males and 19 females) was referred without receiving the NHS because they were treated in the NICU. The hearing of all referred infants was evaluated using impedance audiometry, auditory brainstem response (ABR), and otoacoustic emission (OAE) before six months of age, and follow-up hearing tests were conducted before 12 months of age. We analyzed the prevalence of hearing loss and changes in hearing thresholds.
RESULTS
Prevalence of hearing loss in healthy infants who failed the NHS test and NICU infants
The mean age of the 193 healthy infants who failed the NHS test was 1.9 months at the time of their initial diagnostic hearing tests (Fig. 1). Sixty infants (31%) had normal hearing acuity. We found that 126 infants (65%) experienced SNHL with ABR thresholds >40 dBnHL, 38 with unilateral hearing loss and 88 with bilateral hearing loss. Seven infants (4%) had auditory neuropathy (AN), including five with unilateral AN, and 2 with bilateral AN. The prevalence of SNHL in healthy infants who failed the NHS was 69% (133 out of 193 infants), while the prevalence of AN within the population of infants with SNHL was 5.3% (7 out of 133 infants).
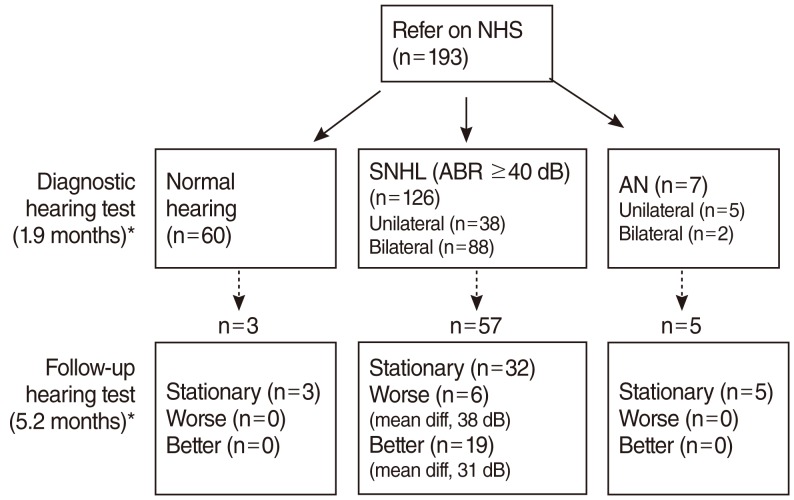
Results of the initial diagnostic hearing test and follow-up hearing tests conducted in infants who could not pass newborn hearing screening (NHS). SNHL, sensorineural hearing loss; ABR, auditory brainstem response; AN, auditory neuropathy; diff, difference. *Values in parentheses indicate the mean age at the time of hearing test.
Of the 133 infants with confirmed hearing loss, only 20% presented risk factors for hearing loss (Fig. 2). Fifty-four percent of the infants with risk factors and 74% of those without risk factors were shown to have confirmed hearing loss. Thus, the presence of risk factors did not increase the prevalence of hearing loss.

Incidence of hearing loss according to the presence of risk factors for hearing loss. The presence of risk factors did not increase the incidence of hearing loss. NHS, newborn hearing screening; SNHL, sensorineural hearing loss; AN, auditory neuropathy.
The mean age of the 51 infants treated in the NICU was also 1.9 months at the time of their initial diagnostic hearing tests (Fig. 3). Thirty-eight infants (75%) showed normal hearing acuity. Twelve infants (24%) had SNHL, 5 with unilateral hearing loss and 7 with bilateral hearing loss. One infant (2%) had AN. The prevalence of SNHL in NICU infants was 25% (13 out of 51 infants). The prevalence of AN within the population of NICU infants was 0.2% (1 out of 51 infants), while that of AN within the population of NICU infants with SNHL was 7.7% (1 out of 13 infants).
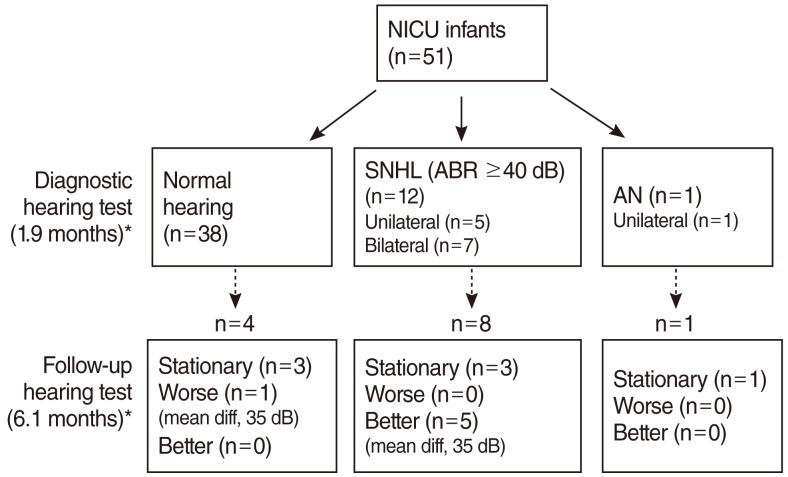
Results of initial diagnostic hearing test and follow-up hearing test performed in infants who were treated in the neonatal intensive care unit (NICU). SNHL, sensorineural hearing loss; ABR, auditory brainstem response; AN, auditory neuropathy; diff, difference. *Values in parentheses indicate the mean age at the time of hearing test.
Changes in hearing thresholds of healthy infants who failed the NHS test
The follow-up hearing tests for healthy infants who failed the NHS test were conducted at the mean age of 5.2 months in 65 infants, 3 with normal hearing, 57 with SNHL, and 5 with AN (Fig. 1). From this group, 6 infants who had had SNHL in the initial hearing tests showed a hearing threshold deterioration of more than 20 dB (mean difference of threshold, 31 dB). In particular, one infant with unilateral mild hearing loss and another infant with bilateral moderate hearing loss progressed to bilateral severe hearing loss. On the other hand, 19 infants who had SNHL showed a hearing threshold improvement of more than 20 dB (mean difference of threshold, 38 dB), and four of them recovered to normal hearing on follow up hearing tests.
Changes in hearing thresholds of NICU infants
Follow-up hearing tests for NICU infants were performed at the mean age of 6.1 months in 13 infants, 4 with normal hearing, 8 with SNHL, and one with AN (Fig. 3). One infant with normal hearing progressed to severe hearing loss. Five infants who had SNHL in the initial hearing tests showed a hearing threshold improvement of more than 20 dB (mean difference of threshold, 35 dB), and four of them recovered to normal hearing.
DISCUSSION
We investigated the prevalence of hearing loss and changes in hearing thresholds during the first year of life in infants who failed the NHS test and in infants treated in the NICU. This investigation provided important epidemiological evidence regarding hearing loss in infants.
The prevalence of hearing loss in healthy infants who failed the NHS test was 69%, i.e., the positive predictive value of NHS, while the prevalence of hearing loss in NICU infants was 25%. These rates were higher than those reported previously. The positive predictive value of NHS was reported to be 30.4% or 14.3% in studies of Korean infants (10, 11), and the prevalence of hearing loss in the population of NICU infants was reported to be 7-9% (8, 12). It is possible that the cohort used in the current study reflected a more at-risk population because it was conducted in a tertiary care hospital and the sample size was small. However, the prevalence of AN in the population of healthy infants with SNHL (5.3%), NICU infants (0.2%), and NICU infants with hearing loss (7.7%) were similar to previous reports. The prevalence of AN in healthy infants with SNHL varied widely from 1.8% to 14.6% (13, 14). The prevalence of AN in the high-risk population varied from 0.2% to 4.0%, while that in NICU infants was higher than in healthy infants (15, 16).
Delayed hearing loss and progressive hearing loss are well-known in infants with risk factors for hearing loss (17, 18). The presence of risk factors increases the possibility of delayed hearing loss to about tenfold (19). Therefore, even if normal hearing is confirmed in the initial hearing test, follow-up tests should be performed for infants with risk factors. In this study, we were able to perform follow-up hearing tests only in 7% (n=7) of the infants who had normal hearing in the initial diagnostic hearing test. Of these, one infant with congenital cytomegalovirus infection showed deteriorated hearing of 70 and 80 dBnHL in each ear in follow-up hearing tests (Fig. 4). Furthermore, six healthy infants who were diagnosed as having SNHL in the initial hearing test showed hearing threshold deterioration of more than 20 dB, and a few infants with mild to moderate hearing loss progressed to severe hearing loss, revealing their candidacy for CI. These findings demonstrates the importance of conducting further hearing tests to examine the presence of delayed hearing loss and progressive hearing loss in healthy infants with SNHL as well as infants with risk factors for hearing loss.
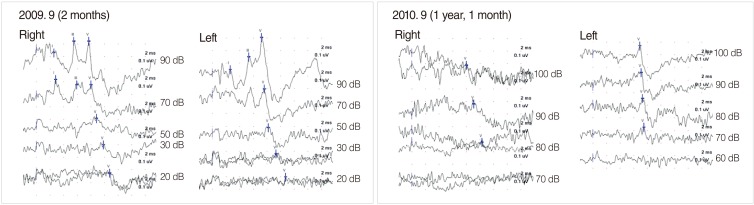
Change in hearing thresholds of an infant with congenital cytomegalovirus infection. Initial auditory brainstem response (ABR) revealed normal hearing thresholds of 20 dBnHL for both ears. Follow-up ABR showed elevation of hearing thresholds to 70 and 80 dBnHL for both ears, respectively.
On the other hand, hearing threshold improvements has also been reported in infants with AN and NICU infants (20-23). Madden et al. (20) observed that 50% of 18 children with AN demonstrated a spontaneous hearing improvement 1 to 15 months after diagnosis with AN, and four children did not require any hearing amplification because of spontaneous improvement. Hearing recovery in infants treated in the NICU has shown to be implicated in cleft palate, hyperbilirubinemia, prematurity, neonatal sepsis, or low birth weight (21-23). Cleft palate is frequently associated with abnormal Eustachian tube function, so otitis media with effusion at the time of initial hearing tests can cause an elevated threshold (21). Therefore, an otoscopic examination or a tympanogram is warranted in order to evaluate middle ear function with the ABR test, and the delay of wave I in the ABR waveform also should be cautiously examined. Neonates with hearing loss and hyperbilirubinemia can recover their hearing with normalization of the serum level of bilirubin, which can cause a transient abnormality of the auditory nerve and brainstem function (22). In severely premature babies, the delayed maturation of the auditory pathway may contribute to a spontaneous hearing threshold recovery (23). In our series, we were able to perform follow-up hearing tests in half (n=71) of the infants (n=146) confirmed to have SNHL or AN in the initial diagnostic hearing tests. Of these, 24 infants (34%) demonstrated hearing threshold improvements of more than 20 dB, and eight of them recovered to normal hearing. In particular, one healthy infant who was diagnosed with profound SNHL at 6 months of age showed a hearing improvement to 70 dBnHL and then 50 dBnHL on a series of follow-up hearing tests (Fig. 5). He was a candidate for CI at 6 months of age, but now, he demonstrates normal development in speech and language ability with assistance by a hearing aid alone.
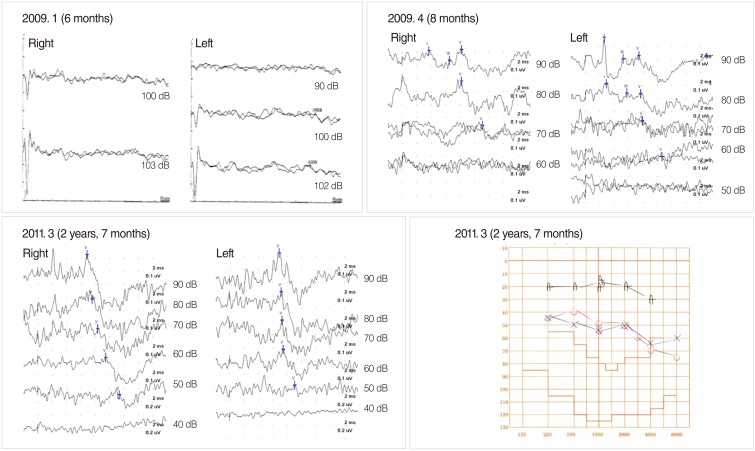
Change in hearing thresholds of a healthy newborn. Initial auditory brainstem response (ABR) revealed that he had profound sensorineural hearing loss. His hearing improved to 70 dBnHL and then 50 dBnHL in follow-up hearing tests.
All newborns should receive hearing screening tests for within 1 month of age, and if indicated, should receive diagnostic hearing tests to confirm the presence of hearing loss before 3 months of age. Appropriate therapeutic options should be selected upon careful analysis of the diagnostic results. Here, we presented the results of follow-up hearing tests performed in infants during the first year of life. Our data showed that hearing acuity easily changes during the first year of life. Therefore, follow-up hearing tests should be mandatory, and therapeutic options including hearing aids and especially CI should be recommended with great caution during this period.
ACKNOWLEDGMENTS
This study was supported by research funds from Dong-A University.
Notes
No potential conflict of interest relevant to this article was reported.