Cochlear Implant Outcomes: A Comparison between Irradiated and Non-irradiated Ears
Article information
Abstract
Objectives
Radiotherapy for head and neck tumors is known to potentially induce sensorineural hearing loss, which is possibly due to damage to the cochlear and/or auditory pathways. Since the success of cochlear implantation depends on a functional auditory nerve, this paper aims to study the hearing outcomes of cochlear implantation in irradiated ears.
Methods
A retrospective study of cochlear implant recipients from our institution who had previously received radiotherapy for head and neck cancers was performed. A control group with cochlear implants who did not receive radiotherapy was recruited. A review of case records, speech discrimination scores (SDS), and a validated subjective questionnaire in the form of the Abbreviated Profile of Hearing Aid Benefit (APHAB) was administered to the study group who fulfilled the inclusion criteria. Global and category scores in both groups were averaged and statistically compared via non-inferiority (NI) testing.
Results
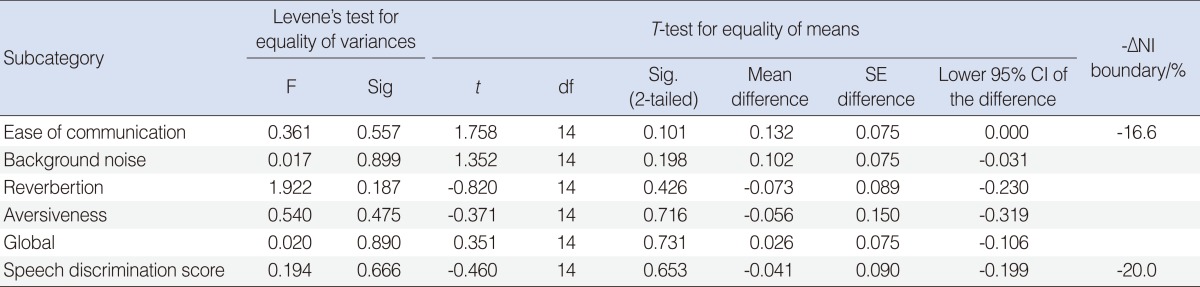
With the control group (n=8) as the reference, the -ΔNI was defined, and a one-tailed lower 95% confidence interval was used for the irradiated group (n=8). The APHAB degree of improvement (%) results were as follows: global, 28.9% (19.32%, -ΔNI=16.3%); ease of communication, 67.0% (58.36%, -ΔNI=37.5%); background noise, 53.2% (44.14%, -ΔNI=26.8%); reverberation, 41.7% (28.85%, -ΔNI=32.7%); and aversiveness, -46.2% (-67.80%, -ΔNI=-56.9%). The SDS was 66.9% (56.02%, -ΔNI=51.0%). From the results, lower 95% confidence interval limits of global APHAB, SDS, ease of communication, and background noise scores of the irradiated group were within the defined -ΔNI boundary and hence are not inferior to the control. The categories of reverberation and aversiveness could not be proven, however.
Conclusion
This study demonstrated marked improvements in hearing measured both objectively and subjectively. The overall hearing outcomes after cochlear implantation for post-irradiated patients were not worse than patients who have had no prior irradiation to ear structures.
INTRODUCTION
Radiotherapy (RT) for head and neck tumors is a commonly used therapeutic modality due to its effectiveness and minimal aesthetic impact. However, given the close proximity of vital structures in the head and neck, such an intervention is not without side effects. These are not limited to the ear structures but may extend to the surrounding central nervous system, vessels, joints, and bones as well as the overlying skin. There exist numerous reports of side effects such as osteoradionecrosis, cranial nerve palsies, and, in the context of this paper, hearing loss.
Sensorineural hearing loss is a well-known side effect of conventional radiotherapy due to damage to the cochlear and auditory pathways, as they are often included in the radiation fields (1). Prospective studies showed that this could result in sensorineural hearing losses that were severe to profound (2). Studies using animal models have demonstrated that high doses of radiation led to destruction of auditory nervous pathways (3). Amidst such well-established evidence, Low et al. (1) showed the absence of retro-cochlear ill effects following therapeutic irradiation for patients with nasopharyngeal carcinoma (NPC) using auditory brainstem response audiometry. This study demonstrated no statistical difference between inter-wave latencies recorded during and after RT as compared to those recorded before RT.
Although it is well known that cochlear implants (CI) have utility in restoring hearing loss from etiologies such as trauma and presbycusis, the effectiveness of cochlear implantation radiation-induced deafness remains unclear. In the current literature, there are only a handful of case reports and case series that suggest cochlear implantation as an effective rehabilitative tool in mitigating hearing loss in patients who received RT to the head and neck (4-7). These observations were consistent with the finding that the retro-cochlear pathways were functionally spared in modern day radiotherapy of head and neck cancers (1). In view of the scarcity of evidence in this aspect, more clinical studies on the feasibility of CI in irradiated ears are warranted.
This non-inferiority study aims to further enhance the belief that CI can serve as a viable option in restoring hearing in deafened patients who had previously received radiotherapy for head and neck cancers. We studied post-implanted patients, comparing a group of patients with previously irradiated ears with a group of controls who have not received radiotherapy before.
MATERIALS AND METHODS
A retrospective review of all patients who had received CI at our institution was conducted. All CI recipients who had a history of having received conventional RT for NPC were identified. For this subset of patients, besides a review of case records, all were assessed by the validated questionnaire of Abbreviated Profile of Hearing Aid Benefit (APHAB) as well as a speech discrimination test. A control group of equivalent patients who had received CI for reasons other than post-irradiation were also recruited to serve as the reference.
Speech discrimination testing was administered aided by a certified team of audiologists and performed in a quiet listening situation in an audiometric booth using a 50 item Arthur Boothroyd (AB) words listing in the appropriate language medium. This was presented using a live voice with the subject seated at a distance of one meter from the tester. The presentation level was at a normal conversational level, and scores were calculated based on the number of correctly repeated phonemes. The validated APHAB questionnaire was explained and administered by the same investigator for all patients in the appropriate language medium.
APHAB
The APHAB is comprised of an inventory of 24 items scored in four six-item subscales. Three of the subscales address speech understanding in various everyday environments: ease of communication (EC, under relatively favorable conditions), listening under reverberant conditions (RV, communication in reverberant rooms such as halls or churches), and listening in background noise (BN, in settings with high background noise levels). The fourth subscale measures the negative reactions to environmental sounds: aversiveness of sounds (AV). Each question was scored accordingly to a seven point Likert scale (for the purposes of this study numbered 0-6), whereby a higher score signifies more problems the subject faces in their daily lives. Of note was that several of the individual APHAB questions have "reversed" answers; these were transposed during analysis so that all larger numeric responses indicated worse performance or more problems. To facilitate ease of calculation, each category was scored upon a subtotal of 36 points; the final global score of all four categories was scored upon a total of 144 points. Their percentages were computed, and a degree of hearing benefit (%) was derived (non aided scores-aided scores). The speech discrimination test uses a validated AB words list whereby the higher the percentage score indicates the better the performance of the patient; in this domain, the aided scores were subtracted from the non-aided scores.
Radiotherapy technique
All patients were treated with six megavolt (6 MV) X-rays from linear accelerators. Chemotherapy was not part of the protocol for any patient. The primary volume covered the nasopharynx, including the Eustachian tube, adjacent parapharynx to the level of the inferior border of C2, and posterior third to half of the nasal cavity and maxillary antra. The brainstem was shielded throughout on the lateral fields, and the inner ear would be at the edge of this shield. A total dose of 66-70 Gy in 2 Gy daily increments was prescribed. The neck received 60 Gy electively, with palpable nodes boosted to 70 Gy.
Statistical method
The mean values and standard deviations for both irradiated and control groups were compared using an independent group T-test to ascertain if there was a significant difference in their degree of hearing benefit. The APHAB and speech discrimination scores (SDS) prior and after CI were also tabulated to further compare the similarity of both groups as well as allow the authors to make a descriptive comparison.
The control group results were used as the reference to ensure uniformity in the geographical context in which the study was conducted. This was also due to the fact that the APHAB was originally developed for hearing aids, and its use in the assessment of CI has largely been confined to small samples in the context of CI fitting strategies (8-10). There are currently no substantial large-scale studies using this clinical utility to ascertain the norm values in current CI users that we can use as a reference.
A non-inferiority (-ΔNI) margin was arbitrarily decided upon and is defined as a degree of hearing benefit (%) change of 16.3 (smallest observable change on the Likert scale) for the APHAB categories and 20% for the SDS based on clinical experience for an observable objective difference. As long as the lower confidence interval of the irradiated group does not exceed the -ΔNI margin, the subcategory is considered to have performed as well as the controls. If the lower confidence interval exceeds this margin, then we cannot conclusively prove the result.
RESULTS
A total of 8 patients (3 females and 5 males) aged 57 to 72 years fulfilled the above criteria and consented to participate in the study. All were diagnosed with NPC, treated with RT from the period of 1979 to 2002, and received CI between the years 2000 and 2010. All patients had a minimum of three years of profound sensorineural hearing loss prior to CI. All patients identified were evaluated at a minimum of one year post-implantation.
With the control group (n=8) as the reference, the -ΔNI was defined, and a one-tailed lower 95% confidence interval was used for the irradiated group (n=8).
Comparison of APHAB and SDS results
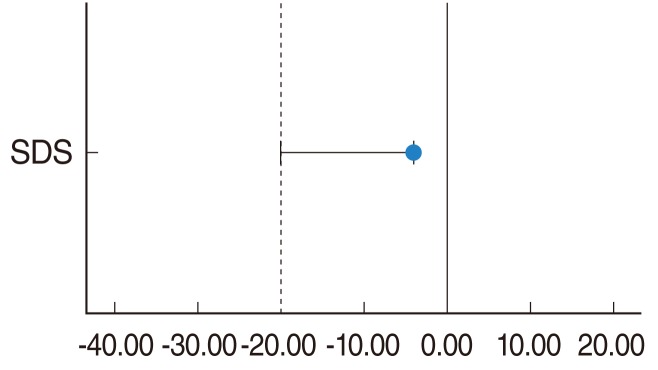
Fig. 1 depicts the mean responses for APHAB and SDS for both the irradiated and control groups in the unaided listening condition prior to their cochlear implantation. The average frequency of problems (represented as percentages) without the use of amplification was similar in both groups, and no significant differences (P>0.05) were observed between the means of the subcategories of BN, RV, AV, global scores and SDS when subjected to an independent samples T-test. There was statistical significance found in the EC scores with T-testing, indicating that there were more problems in the EC domain reported in the irradiated group (X, 97.9%; standard deviation [SD], 5.89) as compared to the control (X, 83.0%; SD, 16.4; t=2.428; P=0.029) with 95% confidence intervals 1.74 and 28.12, respectively. Given the small sample of patients, it would be reasonable to conclude that patients in the irradiated group were largely similar to the control group in most domains except for EC. For the purposes of this study, more emphasis would be placed on the degree of hearing benefit and whether this benefit is similar in both groups.
Mean responses for Abbreviated Profile of Hearing Aid Benefit (APHAB) & speech discrimination scores (SDS) for both the irradiated and control groups in the unaided listening condition prior to their cochlear implantation. EC, ease of communication; BN, background noise; RV, reverberation; AV, aversiveness. Error bars, +/- 1 SD.
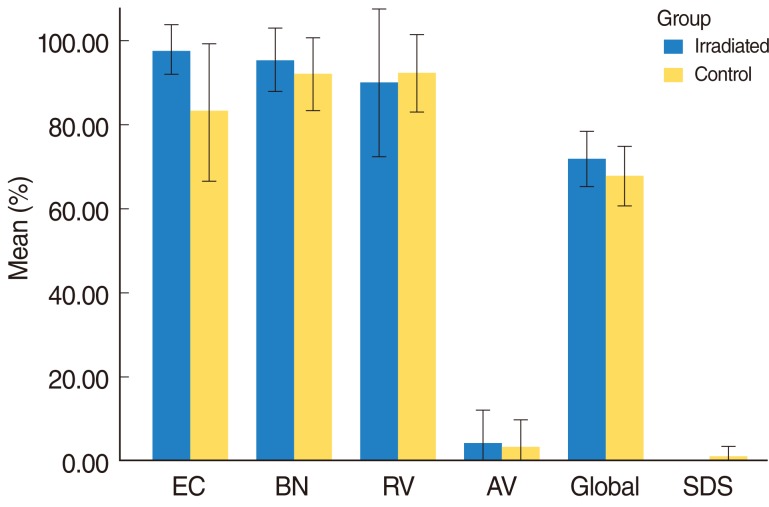
Fig. 2 depicts the mean responses for APHAB and SDS for both the irradiated and control groups in the aided listening condition status post CI with a minimum of 1 year of usage of the CI prior to being inducted into the study. The average frequencies of problems (represented as percentages) between the groups were similar with T-testing that revealed non-significant differences in all sub categories (P>0.05). This suggests that the subjective and objective results were comparable in both groups and that the patients in the irradiated group were able to perform to a level similar to that of the controls.

Mean responses for Abbreviated Profile of Hearing Aid Benefit (APHAB) & speech discrimination scores (SDS) for both the irradiated and control groups in the aided listening condition status post cochlear implants (CI) with a minimum of 1 year of usage of the CI prior to being inducted into the study. EC, ease of communication; BN, background noise; RV, reverberation; AV, aversiveness. Error bars, +/- 1 SD.
Fig. 3 and Table 1 present the comparison of the average benefit for the irradiated and control groups. The average benefit score is given for both groups for each subcategory. As suggested by the aided scores, the data revealed similar mean responses between the two groups in terms of the degree of hearing benefit improvement in all subcategories. T-testing supported this finding, and the comparison of mean hearing benefit scores showed non-significant differences between the two groups (P>0.05).
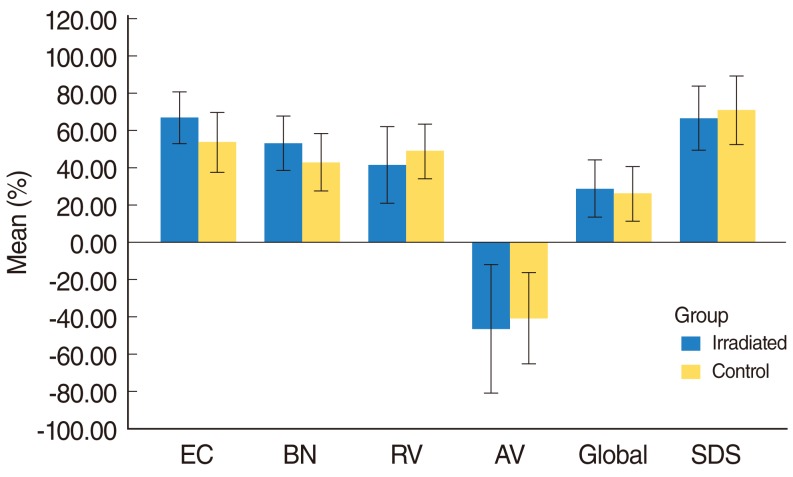
Comparison of the mean degree of hearing benefit for the irradiated and control groups. EC, ease of communication; BN, background noise; RV, reverberation; AV, aversiveness; SDS, speech discrimination scores. Error bars, +/- 1 SD.
A further test of NI was undertaken to further prove that those who suffer from hearing loss due to previously received irradiation have been able to attain a mean degree of hearing benefit that is not worse when compared to the controls. A one-tailed 95% confidence interval was established for all subcategories and compared in Figs. 4 and 5. The subcategories of EC, BN, global, and SDS scores have a mean difference and a lower confidence interval that was within the -ΔNI boundary of -16.6% (-20.0% for SDS). Whereas for the subcategories of RV and AV, despite having a mean difference as compared to controls of -7.29% and -5.55%, respectively, which was well within the -ΔNI boundary, the lower 95% confidence limits of -23.0% (RV) and -31.9% (AV) exceeded the lower limit of -ΔNI. This indicates that for the subcategories of EC, BN, global, and SDS, the irradiated group under study was not worse off compared to controls, but that is not the case for the subcategories of RV and AV. In addition, the T-testing done on the mean differences for all subcategories revealed a P>0.05, indicating no significant differences between the groups.
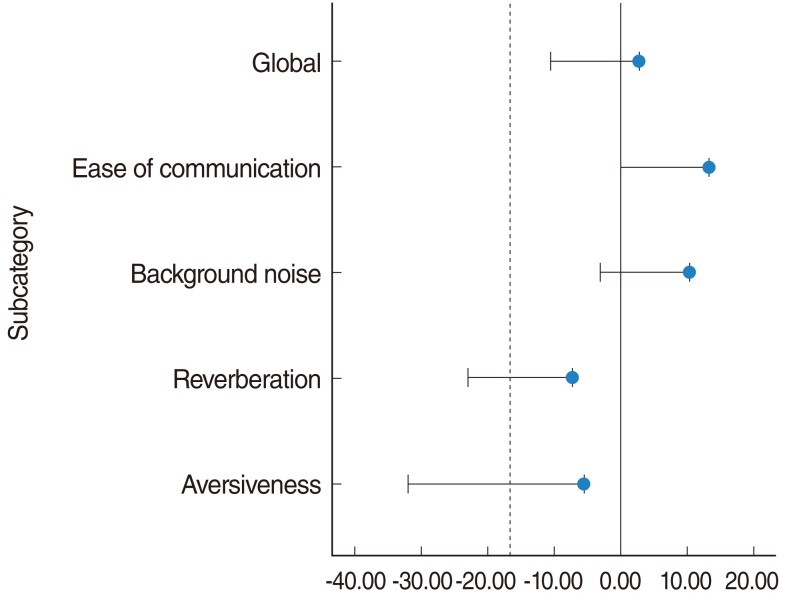
Non-inferiority diagram depicting the mean differences for each subcategory of Abbreviated Profile of Hearing Aid Benefit between the irradiated and control groups.
DISCUSSION
Sensorineural hearing loss as a result of RT treatments to the head and neck region can result with doses as low as 30 Gy (7). In Asians, patients suffering from NPC receiving RT treatments are the largest group at risk for radiation induced hearing loss. Such a phenomenon is common but often ignored, with reported incidences varying up to 54% after RT (11, 12). This is more common in older patients, with a 37% incidence in those over 50 years old (13). Post-irradiation sensorineural hearing loss can occur as early as 3 months to 1 year after treatment, and, as evident from clinical experience, it tends to be progressive, eventually leading to complete hearing loss (2).
Low et al. (1) suggested that there was sparing of the retro-cochlear auditory pathways in a study of 27 patients by demonstrating no statistical difference between inter-wave latencies recorded during and after RT as compared to those recorded before RT. As such, the preservation of retro-cochlear pathways meant that CI could be a viable solution to aid such patients in restoring hearing loss to a reasonable level to that prior to RT.
From our center's experience and through the results of this study, the use of CI in such a group of patients has yielded good responses. However, there are currently no large-scale studies available that could have established the norm values of APHAB scores in non-irradiated CI patients. The closest obtainable results come from a study of 10 patients by Skarzynski et al. (14), in which the APHAB scoring done for this group of partial deafness CI patients reflects a similar degree of hearing benefit, with an average change hovering between 30-40% for all APHAB subcategories except for AV (measures unpleasantness of environmental sounds), which had a cumulative negative change of about 30% over a 12-month period. These results mirror that seen in the controls of this study, which serve to further strengthen the reliability of this reference group. A possible explanation for the negative change in the AV subcategory for both studies could be due to the fact that the patients under study suffer profound hearing loss and would naturally report near zero disturbances in environmental sounds; upon receiving the implant, time is required to get accustomed to the sounds. Another study investigating the use of bilateral versus unilateral CI also utilized the APHAB; however, no raw values were provided, and the scoring method was modified (9). There are larger studies to measure outcomes involving CI in elderly patients for various etiologies not otherwise specified (15, 16). Unfortunately, the APHAB was not employed, and the standard results were only established for the Glasgow Benefit Inventory (GBI) and the Hearing Handicap Inventory for Adults (HHIA), which were not suitable for the purposes of this study. It would thus be reasonable to take the controls recruited in this study as an accurate reference against which the APHAB scores from the irradiated patients can be compared.
Interestingly, the APHAB scores obtained from the controls of this study are similar to the norm values for wide-dynamic range compression-capable hearing aids (17). The 50th percentile established from the degree of hearing benefit were EC (38%), BN (33%), RV (34%), and AV (-13%). In other words, the results obtained in this study fared better on the average compared to those with hearing aids (except for AV).
SDS improvement levels were also similar, indicating that the irradiated group was able to perform to a level comparable to and not worse than that of controls. The controls in this study also performed to superior levels when compared to another study. Orabi et al. (16) reported SDS of 10% at pre-implant levels that improved to 30% nine to twelve months post-implant. Norm values for AB words list testing in CI patients has yet to be established.
Limitations
A retrospective review of a relatively small sample of patients will inherently introduce bias into the study. This is most pertinent in the administration of the APHAB in which candidates have to score the sub-scales according to their performance from memory prior to receiving their CI.
Another point of contention would also be the use of non-inferiority analyses to prove that the irradiated group is not worse off when compared to controls. Given that there are no norm values established for CI users for the APHAB and SDS, the -ΔNI boundary was arbitrarily set as the smallest measurable change for the APHAB and was based on clinical judgment for what is considered reasonable improvement for the SDS.
Due to the constraints of the study, only the objective test of AB word list and subjective questionnaire testing of APHAB were implemented. Other objective testing such as the Consonant-Nucleus-Consonant words, Central Institute for the Deaf sentences, and Hearing in Noise Test sentences were not employed. Other methods that were not used included subjective questionnaires, such as the Glasgow Health Status Inventory Questionnaire, which measures the effect of a hearing problem on the quality of life (overall life, general, physical health, and social support) before and after CI, and the GBI, which assesses the benefit to the psychological, social, and emotional aspects of quality of life affected by impaired hearing to better measure the change in health status brought about by the CI. A wider use of clinical tools can further substantiate the trend reflected by this study. In addition, there are more studies available that use such tools against which a reference can be measured.
These limitations do not undermine the trend demonstrated by this study that CI is clearly able to add to the value of hearing rehabilitation in irradiated patients. In addition, the APHAB is a subjective scoring system; the substantial change in degree of hearing benefit in the study group itself is indicative of a favorable outcome for CI in irradiated ears.
Clinical considerations
In this study group, NPC was the main indication for RT treatment, and the reported 5-year local control rates of NPC in modern series ranged from 81 to 85%, with control rates exceeding 90% for patients with T1 disease (18, 19). Despite the effectiveness of RT as a first line treatment, local recurrence still represents a major cause of mortality and morbidity in advanced stage disease. Magnetic resonance imaging scans are frequently employed to rule out tumor recurrence involving the cerebellopontine angle as well as for tumor surveillance. Magnets from the internal device would need to be removed prior to scanning. Surgical considerations would also have to be thought of in cases in which diagnostic biopsies or excision of recurrent tumors would be necessary. Bipolar diathermy should thus be used for intra-operative coagulation to avoid collateral damage to the CI and the delicate surrounding structures.
The effects of RT on temporal bone structures can pose problems during cochlear implantation. Radiation induced Eustachian tube fibrosis, middle ear effusions, and chronic suppurative otitis media contributing to conductive hearing loss can all complicate CI candidacy. Adhesions that form could also prove to be a challenge through distortion of the normal ear anatomy, especially for the round window niche and the smooth insertion of the electrode array. In addition, trismus due to fibrosis of the temporomandibular joints and adjacent muscles can pose difficulties during anesthetic intubation for surgery. Osteoradionecrosis may result and is of concern as a source of infection. RT also softens the temporal bone and makes the facial nerve vulnerable to iatrogenic damage during temporal bone dissection (20).
Radiation on skin and soft tissues can hamper healing times, and utmost care should be exercised in the design of skin incisions, handling of soft tissue, and avoidance of excessive skin tension during closure. The wound should also be monitored for infections, and preoperative antibiotic coverage should be provided.
In conclusion, this study, performed on a relatively small number of patients, demonstrated marked improvements in hearing measured both objectively and subjectively. The overall hearing outcomes after cochlear implantation for post-irradiated patients were not worse than for patients who have had no prior irradiation to ear structures. These findings should be substantiated by further larger scale studies.
ACKNOWLEDGMENTS
The authors would like to thank Associate Professor Tai Bee Choo; Luc Morris, MD; and Ouyang Hongyue for their kind assistance in the area of statistical analysis.
Notes
No potential conflict of interest relevant to this article was reported.