Early Sensorineural Hearing Loss in Ob/Ob Mouse, an Animal Model of Type 2 Diabetes
Article information
Abstract
Objectives
There have been many studies on the relationship between diabetes mellitus and presbycusis. Microangiopathy and neuropathy that's caused by chronic hyperglycemia may lead to damage to the inner ear. Several clinical studies on humans and animal studies have been performed to investigate the association between diabetes and hearing loss, however, this relationship is still a matter of debate. We investigated the association of diabetes and sensorineural hearing loss in an animal model of type-2 diabetes and obesity (the ob/ob mouse [OM]).
Methods
The auditory brainstem response (ABR) thresholds were obtained in the OM and the wild type mice (C57BL/6J mice) up to 25 weeks after birth. After the animals were sacrificed, their cochleae were retrieved and then subjected to histopathologic observations.
Results
The OM exhibited significantly elevated ABR thresholds at 21 weeks of age, yet the C57BL/6J mice exhibited no significant change until 25 weeks of age. On the histological findings, outer hair cell degeneration and loss of spiral ganglion cells were observed in the middle and basal turns of the OM. On the contrary, no degenerative change was observed until 25 weeks of age in the C57BL/6J mice.
Conclusion
This study suggests that chronic hyperglycemia and obesity may lead to early sensorineural hearing loss.
INTRODUCTION
Diabetes mellitus (DM) is a common metabolic disease that causes various impairments of the body systems. The relationship between diabetes mellitus and hearing function has been studied for a long time, yet there is currently no adequate consensus on this topic. Many clinical studies (1-8) have shown a direct correlation between hearing loss and diabetes, but other studies (9-11) did not identify this association. In addition, several investigators have reported various patterns of hearing loss in diabetic patients. One of these patterns is progressive, gradual bilateral sensorineural hearing loss that especially affects high frequencies and the elderly. This would be similar to presbycusis, but with more severe losses than those expected with normal aging (10, 12, 13). Other studies have reported hearing loss of the low and medium frequencies (6, 14). There are many reasons for the discrepancy in the results from these clinical studies; one reason is that it is difficult to perform histological assessment in human beings. The limited size of the study population and many variables such as the use of drugs, noise exposure, genetic affections and other systemic diseases may contribute to the difficulties of performing human studies.
The effects of diabetes on hearing have been investigated in animal models by using diabetogenic drugs (alloxan or streptozocin) or genetic modifications. Triana et al. study used a rat line that was a genetic model of non-insulin-dependent diabetes mellitus, and they reported that a significant loss of outer hair cells was noted in the diabetic obese rats when compared with the control obese animals (15). However, other authors that used the same animal model reported that diabetes alone did not cause significant basement membrane thickening (16). Ob/ob mice (OM) are hyperphagic, obese, hyperinsulinemic and hyperglycemic, and they have been used as a model for type 2 diabetes and obesity (17). The purpose of this study was to assess the pattern of hearing loss according to aging in OM.
MATERIALS AND METHODS
Experimental animals
Female OM and female wild-type C57BL/6J mice, all 3 weeks old, were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). The mice were kept in specific pathogen-free conditions and in accordance with Samsung Biomedical Research Institute's Principles of Laboratory Animal Care and Guide for the Use of Laboratory Animals. The mice were given free access to food and water and they were placed in a humid environment at a constant temperature with a 12-hr light/dark cycle.
Body weight and the glucose and lipid levels
The body weight and the blood glucose and plasma levels of total cholesterol, triglyceride, LDL cholesterol and HDL cholesterol were determined for every mouse at 5, 9, 13, 17, 21, and 25 weeks of age. Food was not restricted for the blood glucose and lipid analyses. Blood samples were obtained by cardiac puncture and the blood was placed into heparin-coated 1.5-mL capillary tubes, about 0.5 to 1.0 mL from each mouse. The plasma was retrieved after immediate centrifugation for 15 min at 12,000 g at 4℃ and the samples were stored at -80℃ until they were assayed. The plasma levels of total cholesterol, triglyceride, LDL cholesterol, HDL cholesterol and blood glucose were measured by using a clinic chemical system.
Auditory brainstem response (ABR) Recordings
The auditory thresholds were taken for every mouse at the age of 5, 9, 13, 17, 21 and 25 weeks. The auditory thresholds were measured in response to rarefaction clicks (0.1 ms) and tone bursts of 8, 16, and 32 kHz (1 ms rise/fall time, 2 ms plateau and a 11/sec presentation rate) that were generated via an ABR workstation (Tucker Davis, FL, USA), and these were presented via an insert ear phone (Etymotic ER-2, Elk Grove Village, IL, USA). The animals' ears were checked with an operating microscope to ensure that the middle ear was free of infections and fluid. The animals were anesthetized with ketamine (40 mg/kg) and xylazine (5 mg/kg), and then they were placed in an electrically and acoustically shielded sound attenuation booth. Needle electrodes were placed subdermally at the vertex (active electrode) of the skull, in the postauricular region (reference electrode) and on the back (ground electrode). The potentials were filtered (0.3-3 kHz), digitized and averaged with using Tucker-Davis ABR workstation software. The ABR waveforms were averaged (500 sweeps) over a 10-ms time window. Threshold was defined as the lowest intensity for the last visibly repeatable response. The presentation levels of the stimuli varied in 10-dB steps, and they varied in 5-dB steps near the threshold.
Histology
Two or three animals were sacrificed at the age of 5, 9, 13, 17, 21, and 25 weeks, respectively. The animals were deeply anesthetized with ketamine (40 mg/kg) and xylazine (5 mg/kg) and then they were fixed by cardiac perfusion (4% paraformaldehyde in phosphate buffer). After decalcification in EDTA for 2 weeks and embedding in paraffin, 5-µm sections were cut in a paramodiolar plane. Every fifth section was mounted on a glass slide and stained with H&E. For scanning electron microscopy (SEM), the anesthetized animals were sacrificed and the cochleae were removed and the perilymphatic space was perfused with 2.5% glutaraldehyde in 0.1 M cacodylate (CaC) buffer. The following day, the cochleae were rinsed with Cac buffer and then perfused with 1.5% osmium tetroxide. The cochleae were then rinsed with Cac buffer. Under the dissecting microscope, the bony capsule and lateral wall of cochlea were removed to expose the organ of Corti. The cochleae were then dehydrated in increasing concentrations of ethanol, from 70 to 100%, and they were critical point dried. Each specimen was mounted on a SEM stub and sputter coated with 10 nm gold/palladium alloy. The cochleae were viewed and photographed with a Hitachi S-500 scanning electron microscope. Quantitative analysis was not performed for the histology.
RESULTS
Body weight and the glucose and lipid levels
The body weights were remarkably higher for the ob/ob mice after 5 weeks as compared with the C57BL/6J mice. The blood glucose, total cholesterol, LDL and HDL cholesterol, except the TG, were significantly higher in the OM, as compared with the C57BL/6J mice, for all the ages of mice (Fig. 1).
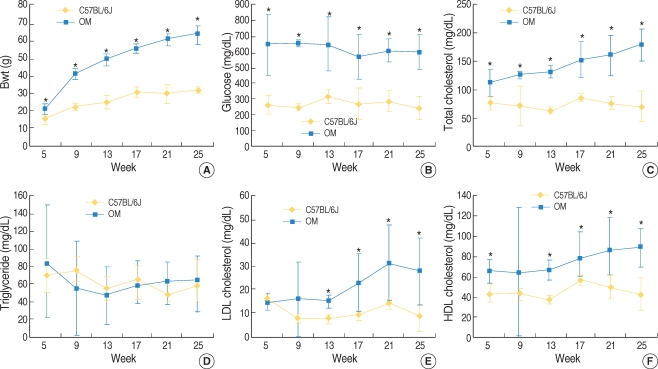
Body weight and the blood glucose and lipid levels in the ob/ob mice (OM) and the age-matched C57BL/6J mice (mean±SD). The body weights of the OM were significantly higher than those of the C57BL/6J mice from 5 weeks of age (A). The blood glucose, total cholesterol, LDL cholesterol and HDL cholesterol levels of the OM were higher than those of the C57BL/6J mice over most of the ages (B, C, E, and F). No significant difference was noted in the triglyceride levels among the two groups (D). *P<0.05, n=12 (OM), 11 (C57BL/6J mice).
ABR thresholds
For the click sounds, a significant difference of thresholds between the two groups was first found at 9 weeks of age. At higher frequencies, a threshold difference was found at earlier ages and the difference became greater according to aging (Fig. 2).
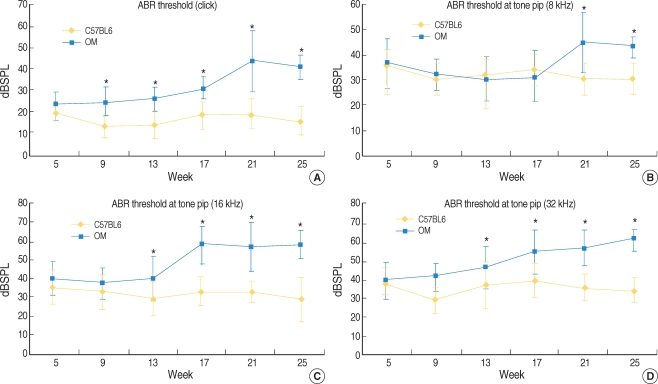
Comparison of the ABR thresholds in the ob/ob mice (OM) and age-matched C57BL/6J mice. For the click sounds, more hearing loss was found from 9 weeks of age in the OM and the differences were greater according to increasing age (A). For the 8 kHz tone pip, significant differences in thresholds were found from 21 weeks of age (B). At 16 and 32 kHz, the differences between the two groups were significant from 13 weeks of age (C and D). *P<0.05, n=12 (OM), 11 (C57BL/6J mice).
Histology
Fig. 3 showed the light microscopic findings of the cochlea. In the C57BL/6J mouse, the outer hair cells (OHCs) and inner hair cells (IHCs) looked intact in the organ of Corti with no obvious neuron loss in the spiral ganglion. However, after 21-weeks of age, the organ of Corti in the cochlear base and middle was significantly degenerated in the OM. On the scanning electron microscopic findings, the IHCs and OHCs looked intact in the C57Bl/6J mice of all ages. On the contrary, degeneration of the OHCs was observed in the 25-week-old OM (Fig. 4).
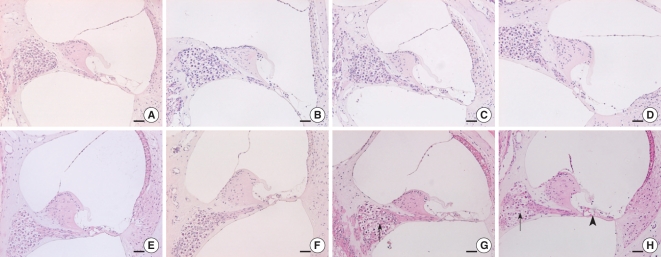
Comparison of the light microscopic findings of the organ of Corti in the C57BL/6J mice and the ob/ob mice (OM) (representative sections). All the sections of the C57BL/6J mice showed a normal organ of Corti and spiral ganglion cells (A: basal turn at 13 weeks of age, B: basal turn at 21 weeks, C: middle turn at 25 weeks and D: basal turn at 25 weeks, respectively). In the OM at 25 weeks of age, the organ of Corti was degenerated (arrow head) and there was significant loss of spiral ganglion cells (arrows) (E: basal turn at 13 weeks of age, F: basal turn at 21 weeks, G: middle turn at 25 weeks and H: basal turn at 25 weeks, respectively). H&E stain, scale bars=100 µm.
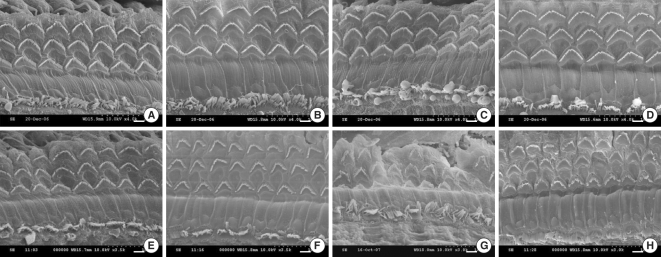
Representative SEM photomicrographs of the organ of Corti from the C57BL/6J mice and the ob/ob mice (OM). The organ of Corti morphology was very well preserved in the C57BL/6J mice of all ages (A-D: mid-basal turns at 5, 9, 21 and 25 weeks of age, respectively). In contrast, some of the outer hair cells were degenerated at 21 and 25 weeks of age, and degeneration of the inner hair cells was found at 25 weeks of age in the OM (E, F: mid-basal turns at 5, 9, 21, and 25 weeks of age, respectively). Scale bars=10 µm.
DISCUSSION
DM is one of the most common metabolic disorders, and its prevalence increases with age. Insulin resistance or type-2 (DM) is often associated with the most commonly occurring metabolic and physiologic problems, including elevated blood pressure, cardiovascular disease, dyslipidemia (high triglyceride levels and low levels of high-density lipoproteins) and high cholesterol levels. The association between hearing loss and DM has been debated since it was first reported by Jordao in 1857 (18). Kakarlapudi et al. (5) reported that sensorineural hearing loss was more common in diabetic patients than in their age-matched controls. Vaughan et al. (19) noted that diabetic patients 60 yr old or younger may show early high-frequency hearing loss similar to early presbycusis, but after the age of 60, the difference in hearing loss between diabetic and nondiabetic patients was reduced. Frisna et al. (4), in their study investigating hearing loss in aged type-2 diabetics and age-matched controls, found a significant difference between both study groups in the inner ear and also a central hearing loss. Interestingly, in that study, the lower frequencies tended to be more affected than the higher frequencies. However, other authors did not find the same association (9-11). There are also controversial reports about the pattern of the hearing loss found in diabetic patients. A small number of studies have assessed human temporal bones from patients with DM. Fukushima et al. demonstrated that cochlear microangiopathy and degeneration of the stria vascularis and cochlear outer hair cells were found in patients with type 2 DM. They also reported a significantly greater loss of outer hair cells in the basal turns in diabetics, as compared with the control group (20). Wackym and Linthicum (21) reported that diabetic sensorineural hearing loss was the result of microangiopathic involvement of the endolymphatic sac and/or basilar membrane vessels. However, they noted no significant differences in the mean estimated percentage of hair cells and stria vascularis cells between the diabetic and nondiabetic patients.
The effects of diabetes on hearing have been investigated in animal models. Smith et al. demonstrated basement-membrane thickening that was consistent with diabetic microangiopathy in the inner ear of streptozocin-induced diabetic rats (22). Raynor et al. (23) observed that the loss of the outer hair cells was greater in the rats with diabetes induced by streptozocin and that were simultaneously exposed to noise. However, drug-induced diabetic animals do not reflect the real physiological mechanism of diabetes in human beings. Thus, genetically modified animals such as Sabra line rats have been more frequently used in research. Nageris et al. used rats of the Sabra line and they demonstrated no statistical difference in the inner and outer hair cells or in the stria vascularis between the genetically induced diabetic rats and the control rats.
Diabetes and obesity are complex genetic diseases that are caused by a combination of a genetic predisposition and environmental factors. The genetic contribution can be either monogenic or polygenic, with polygenic inheritance being the predominant mode of inheritance for human type 2 diabetes and obesity. Although it is rare, single mutations causing monogenic obesity have been identified. Notable among these are mutations of the leptin gene, leading to a complete deficiency of the adipocyte-produced protein hormone leptin. The Lepob mutation arose spontaneously in C57BL/6J mice (commonly referred to as OM) (24). The OM is grossly overweight and hyperphagic, particularly at young ages, and it develops severe insulin resistance. These mice have been have been used as a model for obesity and type-2 diabetes, and they also have dysfunctions, in association with their leptin deficiency, in the immune system and the cardiovascular system, including problems with angiogenesis, supportive tissue repairs, malignancies and reproduction (17). In our study, we compared the ABR thresholds of the OM with age-matched C57BL/6J mice, according to aging. The OM demonstrated early sensorineural hearing loss in comparison with the C57BL/6J mice, and this observation is similar with that from an earlier human study (12). Although quantitative analysis was not performed for the histology, we clearly observed degeneration of the organ of Corti and loss of the spiral ganglion cells in the cochlear basal turn of the OM in comparison with the C57BL/6J mice.
The sequelae of chronic hyperglycemia in diabetes of all phenotypes are divided into the microvascular and macrovascular complications. Microvascular disease causes blindness, renal failure and neuropathy, and diabetes-accelerated macrovascular disease causes an excessive risk for myocardial infarction, stroke and lower limb amputation. The link between chronic hyperglycemia and vascular damage has been established by four independent biochemical abnormalities: increased polyol pathway flux, increased formation of advanced glycation end products, activation of protein kinase C and increased hexosamine pathway flux. In the cochlea, Smith et al. demonstrated basement-membrane thickening in the cochlea, which is consistent with diabetic microangiopathy in the inner ear of IDDM rats (22). Also in a study on human temporal bone, the walls of the vessels of the basilar membrane and stria vascularis in diabetics were significantly thicker than those of the controls (20). Although microangiopathy is an important factor in the cochlear disease of diabetic patients, other precipitating factors such as oxidative stress and apoptosis due to a hyperglycemic state, noise and hypertension can work synergistically to cause the observed pathologic changes in the stria vascularis and the outer hair cells (20).
We observed early loss of the spiral ganglion cells in the OM. Raynor et al. (23) and Ishikawa et al. (25) previously reported the loss of spiral ganglion cells in an animal model of diabetes. However, Fukushima et al. noted no significant difference in the number of spiral ganglion cells or inner hair cells between the diabetics and controls in a human temporal bone study (20). Although OM have been used as an animal model for diabetes, some aspects of this animal are different from human type-2 diabetes. Early obesity in OM can be reversed almost completely, even in adult animals, by exogenous leptin or transfection with the leptin gene. There are also cases with leptin deficiency in obese humans, but this is uncommon and early obesity in type-2 diabetes patients is not usual (17). In addition, uncontrolled severe hyperglycemia as was seen in the OM in this study is not common in human patients. Severe obesity and dyslipidemia also might additively affect cochlear degeneration in the OM, as compared to type 2 diabetic humans. The earlier development of diabetes and obesity in OM may influence the normal development of auditory function, as compared to human type 2 diabetes. However, considering that the P18-20 mouse usually shows adult-like mature ABR waveforms (26), and that the OM showed later elevation of the ABR thresholds in our study, acquired hyperglycemia and obesity appeared to contribute much more to cochlear degeneration than do any genetic or developmental alterations. Obesity may also lead to a decrease in Na and K-ATPase in the nerves of patients with diabetic angiopathy. The Na and K-ATPase levels of hyperglycemic-hyperinsulinemic OM were recently reported to be reduced in the liver and kidney, as compared with the lean control animals (27). So, the loss of spiral ganglion cells in the OM might be caused by hyperglycemia and severe obesity.
CONCLUSIONS
We demonstrated that OM, an animal model for type-2 diabetes and obesity, showed earlier sensorineural hearing loss as compared with the C57BL/6J mouse. We also observed earlier degeneration of the organ of Corti and the spiral ganglion cells on the histology. This study suggests that severe hyperglycemia and obesity may lead to early cochlear degeneration.
ACKNOWLEDGEMENT
This work was supported by grants from the Korea Science and Engineering Foundation (KOSEF, R01-2005-000-10739-0) funded by the Korean Government.