Expression Profile of Fas-Fas Ligand in Spiral Ganglion Cells During Apoptosis
Article information
Abstract
Objectives
To examine the expression profile of Fas-Fas ligand (FasL) during glutamate (Glu)-induced spiral ganglion cell (SGC) apoptosis.
Methods
Cultured SGCs were treated with 10-mM, 25-mM, and 50-mM concentrations of Glu and incubated for 24 or 48 hours. The expression intensity of FasL, Fas, caspase 3, and morphology of single SGC were evaluated using immunofluorescence staining.
Results
In semiquantitative analysis of the Glu-treated SGC, FasL, and caspase 3 expression intensity were increased with concentration- and time-dependent manner. Fas expression intensity did not change with different concentration at 48 hours. In morphologic analysis of the Glu-treated SGC, number of apoptotic cells were increased with concentration- and time-dependent manner.
Conclusion
FasL was expressed in apoptotic SGCs, suggesting that the Fas-FasL signaling pathway may be involved in the Glu-induced apoptosis of dissociated SGCs.
INTRODUCTION
Acoustic stimulation is converted to electric stimulation in auditory hair cells and transmitted to spiral ganglion cells (SGCs). Thus, the survival of SGCs is critical for the preservation of hearing function.
Glutamate (Glu) is the primary amino acid neurotransmitter at the synapses between the inner hair cells and the dendrites of type I SGCs [1]. Various ototoxic agents, such as noise, aminoglycoside, and ischemia, can induce cochlear damage and subsequent secondary apoptosis of SGCs. SGC apoptosis may be triggered by excessive release of Glu into the synaptic cleft or overstimulation of its membrane receptors, also called excitotoxicity. Previously, various animal models have been used to study excitotoxicity [2,3]. However, few in vitro models of cultured SGC apoptosis have examined the time course and different concentrations of Glu exposure.
The activation of procaspase in apoptosis is triggered by two distinct pathways: cell death stimuli from intracellular stresses (intrinsic or mitochondrial pathways), and cell death via a receptor-mediated extrinsic pathway, such as Fas/CD95 from the cell membrane [4]. The Fas-Fas ligand (FasL) system is the best characterized member of the extrinsic pathway family in apoptosis. In our previous study, we demonstrated that the Fas-FasL signaling pathway was involved in the secondary apoptosis of SGCs induced by gentamicin [5].
To develop therapeutic strategies for the protection of SGCs from the secondary degeneration process, the mechanism underlying SGC apoptosis must be understood. However, little is known about the interaction of Glu and the expression of Fas-FasL in SGCs during apoptosis.
This study was performed to examine the expression profile of Fas-FasL during Glu-induced SGC apoptosis.
MATERIALS AND METHODS
Animals
Dissociated cell cultures of SGCs were prepared using 24 ears from 2-5-day-old guinea pigs (n=12), each weighing about 100 g (Samtako Bio Korea, Osan, Korea). The animals were sacrificed under general anesthesia with a mixture of ketamine (0.1 mL/100 g body weight; Sanofi-Ceva, Dusseldorf, Germany) and Rompun (0.05 mL/100 g body weight; Bayer, Leverkusen, Germany). After decapitation of the guinea pigs, the cochleae were removed from the temporal bone. SGCs were microdissected from the modiolus of the cochlea and transferred to a cell culture dish. All experimental protocols were approved by the Animal Research Committee, Dong-A University College of Medicine (Busan, Korea). Animal care was performed under the supervision of the Institute of Laboratory Animals, Dong-A University College of Medicine.
Dissociated cell cultures and induction of apoptosis with Glu
The spiral ganglion was identified, separated from the modiolus, and chemically digested in 1 mg/mL collagenase in calcium/magnesium-free Hank's buffered solution at 37℃ for 1 hour. Separated SGCs were treated with a 0.05% trypsin (Gibco, Grand Island, NY, USA) at 37℃ for 20 minutes, and the enzymatic action of trypsin was terminated by the addition of 10% fetal bovine serum (FBS; Gibco). SGCs were then dissociated by gentle shaking for 2 minutes, followed by two or three triturations using a fire-polished Pasteur pipette. The cell pellets obtained after centrifugation were resuspended in Dulbecco's modified Eagle's medium (DMEM) containing 10% FBS and cultured for 2 days. To enhance the SGC population, cells were cultured in DMEM containing 1% FBS with human neurotrophin-3 (NT-3; 100 ng/mL; Sigma-Aldrich, St. Louis, MO, USA), brain-derived neurotrophic factor (BDNF; 100 ng/mL; Sigma-Aldrich), and transforming growth factor-β (TGF-β; 4 ng/mL; Sigma-Aldrich).
The cells were plated onto a Lab-Tek chamber slide coated with poly-D-lysine (100 µg/mL; Sigma-Aldrich) and laminin (2.5 µg/mL; Sigma-Aldrich) and cultured for 1 day at 37℃. Depending on the experimental group, SGCs were incubated for 24 or 48 hours in 10 mM, 25 mM or 50 mM Glu (Sigma-Aldrich) and fixed with 4% paraformaldehyde.
Immunofluorescence staining
The dissociated cells on the slides were blocked with phosphate-buffered saline (PBS) containing 0.3% Triton X-100. Anti-active caspase-3 antibody (Promega, Madison, WI, USA) with Tuj1 (Neuron-specific class III beta-tubulin) antibody (BD Biosciences, Franklin Lakes, NJ, USA), FasL antibody (BD Biosciences), or neurofilament (NF) antibody (BD Biosciences) diluted 1:500 in PBS containing 5% bovine serum albumin was applied to the slides and incubated for 24 hours at 4℃. After washing with PBS three times, the slides were incubated with fluorescein isothiocyanate-conjugated secondary antibody (Chemicon, Temecula, CA, USA) for 2 hours. Hoechst 33258 (4 µg/mL; Sigma-Aldrich) was used for counterstaining at room temperature for 15 minutes [6].
Assessment of cell death and statistical analysis
Cells were analyzed using a confocal laser-scanning microscope (LSM510; Carl Zeiss, Germany). The criteria of apoptosis were loss of neurites, loss of the typical round or elliptical shape, and cell shrinkage.
The cell death ratio was evaluated and compared among the control and treatment groups.
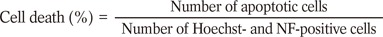
The cell death ratio was evaluated for each incubation period and concentration of Glu. The paired t-test was used to compare cell death between the 24- and 48-hour incubation groups. One-way analysis of variance was used to compare cell death according to Glu concentration. Differences were considered significant when P-values were less than 0.05 (SPSS ver. 15.0; SPSS Inc., Chicago, IL, USA).
Quantitative analysis of the relative intensities of FasL, and active caspase-3
The relative intensities of immunofluorescence staining of FasL, and caspase-3 were evaluated in 10 randomly selected 30×30-µm2 areas from each sample. The intensity of fluorescence was divided into five grades ranging from 0 to 250 (lowest intensity, 0 to 50; highest intensity, 200 to 250). Relative intensity was quantified by counting the number of pixels corresponding to each intensity unit [7]. One-way analysis of variance was used to compare intensity of fluorescence according to Glu concentration. Differences were considered significant when P-values were less than 0.05 (SPSS ver. 15.0; SPSS Inc., Chicago, IL, USA).
RESULTS
Dissociated cell cultures and induction of apoptosis with Glu
Dissociated SGCs showed round nuclei, elliptical cell bodies, and multiple neurites. Upon Glu-induced apoptosis of dissociated SGCs, the neurites were constricted or disappeared after Glu treatment. Apoptotic SGCs showed condensation of the nucleus or fragmentation, as demonstrated by Hoechst staining (Fig. 1).
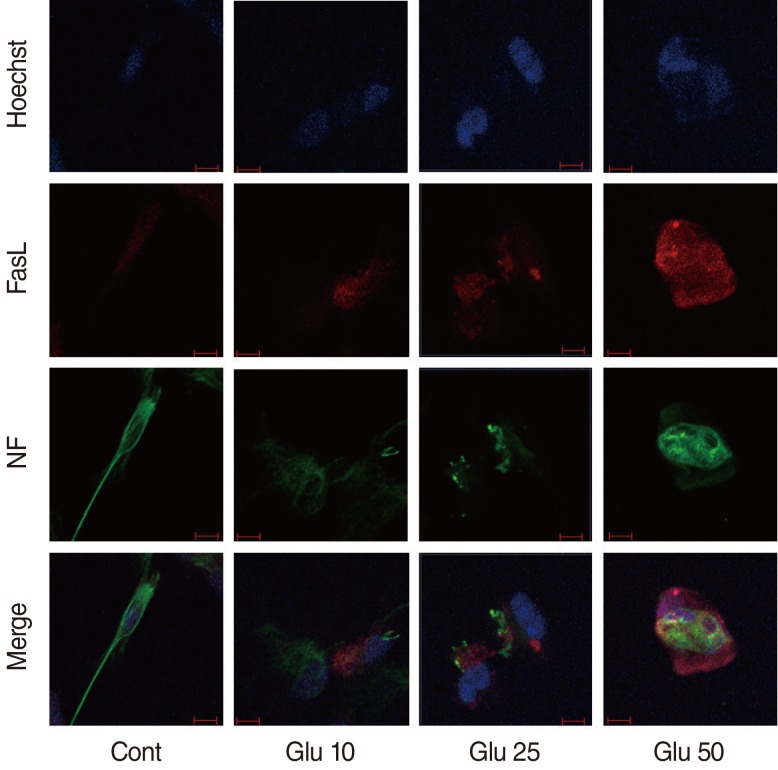
Confocal microscopic images of cultured spiral ganglion cells (SGCs) in control and Glutamate (Glu)-treated group at 24 hours. Non-apoptotic SGCs were showed in control group. Typical apoptotic SGCs showed nucleus condensation or fragmentation at Hoechst staining in the treatment groups. Expression of Fas ligand (FasL) in apoptotic SGCs increased in a dose-dependent manner. FasL increased on cell body, especially around nucleus. Neurites were disappeared and cell body was not elliptical shape with neurofilament staining. Cont, control; Glu 10, 10 mM Glu; Glu 25, Glu 25 mM; Glu 50, 50 mM Glu; NF, neurofilament; red scale bar, 10 µm (×200).
Assessment of cell death and statistical analysis
The apoptotic nuclei from NF-positive cells were counted using a confocal microscope, and this value was compared with the apoptotic ratio following Glu treatment. The ratio of apoptotic cells increased significantly in a dose-dependent manner in Glu-treated SGCs at 24 hours (P<0.05). At 48 hours after Glu treatment, the ratio of apoptotic SGCs increased significantly in each treatment group compared with controls (P<0.001). However, no significant difference among treatment groups was observed. More apoptotic Glu-treated SGCs were observed at 48 hours than at 24 hours after treatment with the same Glu concentration. The cell death ratio of SGCs differed significantly between 24 hours and 48 hours in the 10-mM and 25-mM treatment groups (P<0.001) (Fig. 2).
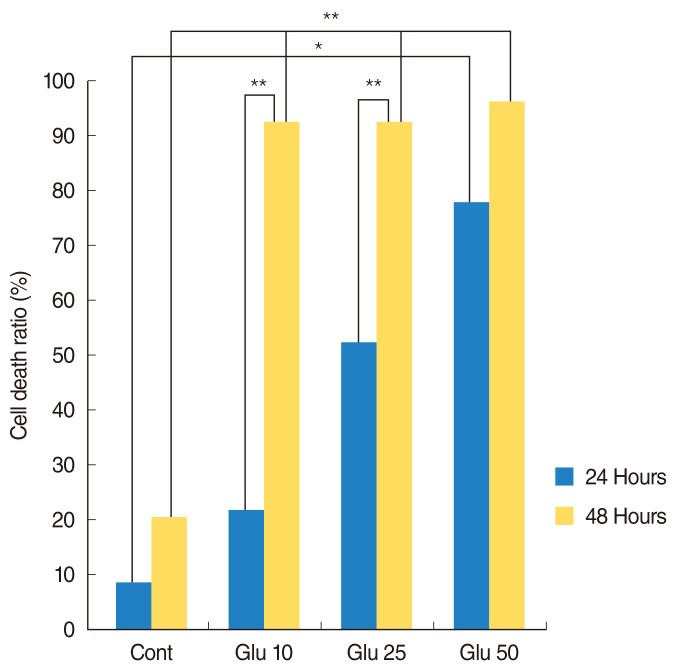
Assessment of cell death. The ratio of apoptotic cells increased significantly in a dose-dependent manner in Glutamate (Glu)-treated spiral ganglion cells (SGCs) at 24 hours (P<0.05). At 48 hours after Glu treatment, the ratio of apoptotic SGCs increased significantly in each treatment group compared with controls (P<0.001). Cont, control group; Glu 10, glutamate 10 mM; Glu 25, glutamate 25 mM; Glu 50, glutamate 50 mM; number of field in each group, 10. *P<0.05. **P<0.001.
Semiquantitative analysis of the relative intensity of FasL in apoptotic cells
SGCs were cultured and immunostained with a FasL antibody (Fig. 1).
Relative intensity of FasL staining at 24 hours after treatment
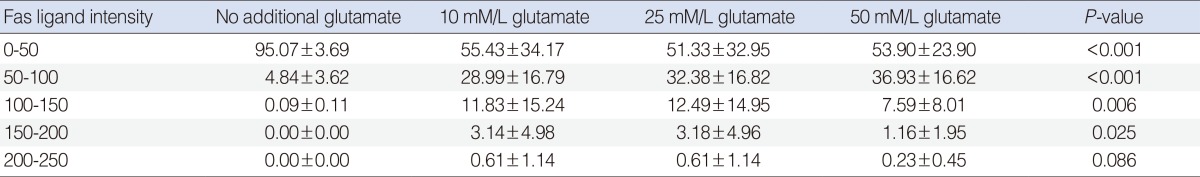
The relative intensity of FasL staining increased in the Glu-treated SGCs (Table 1). This increase was dose-dependent at intensity units of 50-100. The difference in relative intensity was more apparent in the 50 mM Glu-treated SGCs.
Relative intensity of FasL staining at 48 hours after treatment
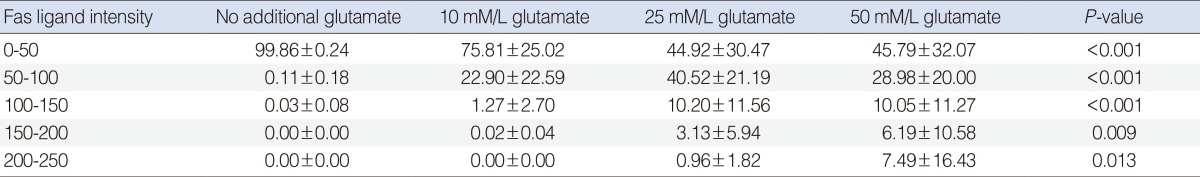
The relative intensity of FasL staining increased in the Glu-treated SGCs. In the semiquantitative analysis, the control group showed the greatest increase in relative intensity at intensity units of 0-50. No significant difference was observed among Glu-treated SGC groups at intensity units of 0-50. The control group showed the lowest level of relative intensity. The relative intensity increased in a dose-dependent manner in the Glu-treated SGC groups at intensity units of 50-100. In SGCs treated with 10 mM and 25 mM Glu, the relative intensities were similar at the intensity units of 100-150 and 150-200, respectively. In SGCs treated with 50 mM Glu, the relative intensity was lower than that observed in SGCs treated with 10 mM or 25 mM Glu at the intensity units of 100-150 and 150-200, respectively (Table 2).
Relative intensity of FasL staining in Glu-treated SGCs at 24 hours and 48 hours for each Glu concentration
A higher relative intensity of FasL staining was observed in Glu-treated SGCs at 48 hours than at 24 hours. The relative intensity of FasL staining in the control group was similar at all intensity grades (Tables 1, 2).
Semiquantitative analysis of the relative intensity of active caspase-3 staining in apoptotic SGCs
Cultured SGCs were double-immunostained with anti-active caspase-3 antibody and Tuj1 antibody. Active caspase-3, a known marker of apoptosis, was expressed in Tuj1-positive cells. The intensity of active caspase-3 staining was increased in a dose-dependent manner in Glu-treated SGCs (Table 3).
DISCUSSION
SGCs are located in Rosenthal's canal within the modiolus of the cochlea. They comprise two subpopulations of neurons, the large type I neurons, representing approximately 95% of the afferent auditory neurons. Cultured postnatal SGCs undergo cell death after trophic factor deprivation [8-10]. Trophic factors important for the survival of cultured SGCs include BDNF and NT-3. NT-3 seems to be the survival factor for type I spiral ganglion neurons, and BDNF is necessary for type II neurons [11]. TGF-β is also an important regulator of neuron survival. TGF-β can dramatically increase the potency of neurotrophins, fibroblast growth factor-2, ciliary neurotrophic factor, and glial cell line-derived neurotrophic factor [12]. NT-3 and BDNF, acting in synergy with TGF-β, have been shown to promote auditory neuron survival in dissociated cell cultures of early postnatal rat SGCs [13]. In this study, NT-3 and BDNF, the survival factors of type I and II spiral ganglion neurons, were added to all experimental SGC groups to prevent apoptosis by trophic factor deprivation. TGF-β was added to all experimental groups to increase the potency of the survival factor.
In this study, the Glu-induced neurotoxicity of SGCs was examined (Fig. 1). Glu is the major neurotransmitter at the synapse between the inner hair cell and the dendrite of the SGCs. Three types of Glu receptors are present in the cochlea: N-methyl-D-aspartic acid (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA), and kainate in type I primary auditory neurons [14]. Excessive and nonphysiological release of Glu into the synaptic cleft leads to swelling and destruction of the afferent nerve terminals, and reduces the number of type I spiral ganglion neurons in the developing cochlea. Excitotoxicity also leads to production of damaging free radicals and other enzymatic processes contributing to apoptosis. Reactive oxygen species (ROS) and the c-Jun n-terminal kinase signaling pathway have been found to be involved in the secondary apoptosis of SGCs, and the Fas-FasL signaling pathway was induced by ROS generation [15]. The present study is the first to directly show the interaction of Glu and the expression profile of Fas-FasL in SGCs during apoptosis.
Our results showed that exposure to nonphysiologic and excessive Glu can induce SGC apoptosis, which increased in a dose- and time-dependent manner (Figs. 1, 2).
Two major pathways of apoptosis have been identified in neuronal cells: the receptor-mediated pathway initiated by ligand binding to death receptors, such as Fas and tumor necrosis factor (TNF) receptor 1; and the mitochondrial pathway initiated by translocation of cytochrome c [16]. Several molecules, including Glu, caspase-3, the Bcl-2 family, and calpain, are involved in the apoptosis of SGCs. However, the precise mechanism and signaling pathway for SGC apoptosis remain unclear [17-19]. Fas (CD95) is a member of the family of death receptors that initiate apoptosis, especially in the immune system, by recruiting Fas-associated death domain proteins, procaspase-8, and procaspase-3, which form after binding to the cognate ligand (FasL or CD95L) [20]. However, little is known about the role of the Fas-FasL system in the inner ear. In the extrinsic pathway, death receptors such as Fas can initiate apoptosis upon ligand binding. FasL is a trimetric type II membrane protein of approximately 37 kD and belongs to the TNF superfamily. The Fas-FasL system is the best characterized member of the extrinsic pathway family. When FasL binds to Fas on target cells, caspases are activated and the cells die through programmed cell death. The Fas-FasL signaling pathway is involved in the secondary apoptosis of SGCs by aminoglycoside. We performed immunofluorescence staining to investigate the expression of FasL. Our results showed a selective increase of FasL expression in Glu-induced apoptotic SGCs. FasL increased in a dose-dependent manner in Glu-treated SGCs at 24 hours after treatment (Table 1). This result showed a correlation between cell viability and FasL expression (Fig. 2, Tables 1, 2). FasL expression, which has been implicated in apoptosis induction, increased in apoptotic SGCs. Although the precise mechanisms involved in this signaling have not been clarified, this study suggests that the Fas-FasL system may play a role in Glu-induced SGC apoptosis. The apoptosis of SGCs is likely to be a result of the apoptotic Fas-FasL signaling pathway.
Activation of caspase-3 is involved in receptor- and mitochondria-mediated apoptotic pathways. In our study, active caspase-3 increased in Glu-induced SGC apoptosis in a dose-dependent manner, suggesting that Glu increased the expression of FasL, activated caspase-3, and induced SGC apoptosis.
Limitation of this study is a preliminary work with only semiquantitative data using immunofluorescence staining. Future study should be conducted with quantitative evaluation of gene expression and final protein such as real time PCR and western blot analysis. This study demonstrated that excessive Glu induced apoptosis of dissociated SGCs, and suggested that the Fas-FasL signaling pathway is involved in the Glu-induced apoptosis of SGCs (Fig. 3).

Schematic figure for the conclusion. Excessive noise, ototoxic drugs and cochlear ischemia lead to excessive and nonphysiological release of Glutamate (Glu) into synaptic cleft. It leads to induce spiral ganglion cell (SGC) apoptosis and the Fas-Fas ligand signaling pathway is involved in the Glu-induced apoptosis of SGCs.
In conclusions, Glu induced apoptosis in dissociated SGCs in a dose- and time-dependent manner. FasL was expressed in apoptotic SGCs, suggesting that the Fas-FasL signaling pathway may be involved in the Glu-induced apoptosis of dissociated SGCs.
ACKNOWLEDGMENTS
This work was supported by the Dong-A University research fund.
Notes
No potential conflict of interest relevant to this article was reported.