Dexamethasone Induces Apoptosis of Nasal Polyp-Derived Tissue Cultures Through JNK and p38 MAPK Activation
Article information
Abstract
Objectives
Glucocorticoids, such as dexamethasone (DEX), increase apoptosis in a variety of white cells in nasal polyps and apoptosis is an important factor in the resolution of inflammation. However, the mechanism of glucocorticoids induced apoptosis in nasal polyp remains unclear. In this study the authors evaluated which pathways were engaged in apoptosis induced by DEX in an ex vivo model of nasal polyps.
Methods
Nasal polyp tissues were cultured using an air-liquid interface method. Cultures were maintained in the absence or presence of DEX (10 or 100 µM) for 24 hours. To investigate the involvement of the apoptotic signaling pathways in nasal polyp, such as caspase cascades, Fas-FasL signaling pathway, mitochondrial pathway and p38 mitogen-activated protein kinase (MAPK)/JNK pathway, the authors performed reverse transcription-polymerase chain reaction and Western blotting.
Results
The expression ratios of FasL, activated form of caspase-8, caspase-9, and caspase-3 were significantly higher in DEX-treated polyps (P<0.01). In the Bcl-2 family expression, the anti-apoptotic molecules, Bcl-2 and Bcl-XL decreased, but pro-apoptotic molecules, Bax increased, and Bid and Bad were activated. In the conventional MAPKs, JNK, and the phospho-p38 MAPK were significantly higher, but phospho-extracellular signal-regulated kinase (ERK)1/2 was significantly lower in DEX-treated polyps (P<0.01).
Conclusion
DEX induces apoptosis of nasal polyp via caspase cascades, Fas-FasL signaling pathway, mitochondrial pathway and p38 MAPK/JNK pathway.
INTRODUCTION
Nasal polyposis, one of the major causes of the chronic rhinosinusitis, is a common disease with a high recurrent rate. It is characterized by morphological changes, such as lining epithelial hyperplasia and metaplasia, inflammatory cells infiltration and stromal fibrosis and edema. Even though the pathogenesis of nasal polyps is not yet clearly understood, it is suggested that the causes are multifactorial, and allergic and inflammatory responses are implicated in polyp formation. In this regard, systemic and topical steroids have been used successfully in the treatment and prevention of nasal polyps. However, the exact mechanism of action is not certain.
Glucocorticoids, such as dexamethasone (DEX), are the most widely prescribed anti-inflammatory drugs. They have been demonstrated to down-regulate pro-inflammatory cytokines and adhesion molecules that attract and activate eosinophils in nasal polyps [1]. They act by binding to the intracytoplasmic receptor, glucocorticoid receptor (GR). The glucocorticoid-GR complex translocates to the cell nucleus, and directly binds to DNA at a specific site, named glucocorticoid response element (GRE), becoming capable of stimulating or inhibiting gene expression [2]. GR-mediated inhibition of a number of pro-inflammatory response genes occurs through synthesis of various anti-inflammatory proteins, as well as by suppression of pro-inflammatory transcription factors, such as NF-κB and AP-1 [3].
Recent research demonstrated that apoptosis in inflammatory cells is an important factor in the resolution of inflammation [4]. Glucocorticoids increased apoptosis in a variety of polymorphonuclear cells, such as eosinophils, T lymphocytes and fibroblasts in nasal polyps [5,6]. The mechanism of glucocorticoids-induced apoptosis performed in lymphoid cells, like thymocytes, has been identified through in vitro studies, however the exact signaling pathway for glucocorticoids induced apoptosis in nasal polyp remains unclear. Every step in the apoptotic cascade is tightly controlled by a complex regulatory network. However, the role of some regulators in apoptosis is controversial, depending on cell type, the nature of the death stimulus, the duration of its activation and activity of other signaling pathways [7].
In this study, the authors evaluated which pathways were activated, which regulators were engaged and what were their roles in apoptosis induced by DEX in an ex vivo model of nasal polyps.
MATERIALS AND METHODS
Materials
Nine subjects with nasal polyps were recruited at the Department of Otorhinolaryngology, Inje University Busan Paik Hospital, Busan, Korea (Table 1). Informed consent was obtained from each patient, and the study was approved by the local ethics committee. Individuals were diagnosed with nasal polyps based on the minimal criteria for chronic rhinosinusitis with nasal polyps [8].
Nasal polyps were removed from the middle meatus at the beginning of the surgical procedure. No patients had been treated with steroids (systemic or topical), nonsteroidal anti-inflammatory drugs, antihistamines or macrolide antibiotics for the 4 weeks prior to biopsy.
Organ culture of nasal polyp
Nasal polyps were cultured using air-liquid interface method [9]. Nine nasal polyps were cut using blades into 2-3 mm3 pieces under sterile conditions. Tissue fragments were washed three times with phosphate-buffered saline containing an antimycotic (5 µg/mL fungizone) and an antibiotic (300 µg/mL penicillin G), and then rinsed with 98% Dulbecco's minimum essential medium (DMEM) supplemented with calf serum (10%) and gentamicin (20 µg/mL). To determine the effects of DEX treatment on nasal polyps, tissues were next saturated for 1 hour in culture medium (DMEM+10% calf serum+10 µg/mL gentamicin) in the presence or absence of DEX (10 or 100 µM). Tissue fragments were then placed on hydrated 1×1 cm gelatin sponge (Spongostan; Johnson & Johnson, San Angelo, TX, USA) with the mucosa facing upward and the submucosa downward. Each sponge was placed in a well of a 6-well plate containing 3 mL of culture medium so that the mucosa was above the liquid phase. The plates were then placed in a humidified incubator and maintained at 37℃ in a 95% air/5% CO2 atmosphere under continuous shaking at 15 rpm for 24 hours. Where specified, DEX (10 or 100 µM) was added to culture medium. Subjects were divided into three groups: (1) non-treated group (nasal polyps were saturated for 1 hour in culture medium and then organ-cultured for 24 hours in culture medium); (2) 10 µM DEX-treated group (nasal polyps were saturated for 1 hour in culture medium containing 10 µM DEX and then organ-cultured for 24 hours in culture medium containing 10 µM DEX); and (3) 100 µM DEX-treated group (nasal polyps were saturated for 1 hour in culture medium containing 100 µM DEX and then organ-cultured for 24 hours in culture medium containing 100 µM DEX).
Reverse transcription-polymerase chain reaction (RT-PCR)
After drug treatment, nasal polyps were cut into small pieces with a sterile surgical blade and homogenized in liquid nitrogen. Total RNA was isolated using RNeasy mini kit (QIAGEN, Hilden, Germany) according to the manufacturer's instructions and cDNA was produced using RT premix (Bioneer, Daejeon, Korea). PCR amplification was performed using specific primer sets (Bioneer) for FasL, Bcl-2, Bax, and Bad. For control, a specific primer set for β-actin was used, which yielded a 200-bp product. Primer sequences of each gene used in this study were listed in the Table 2. PCR (25 cycle; 20 seconds at 94℃, 10 seconds at 60℃, 30 seconds at 72℃) was performed using AccuPower PCR premix (Bioneer). PCR products were analyzed by agarose gel electrophoresis and visualized with ethidium bromide under UV light using Multiple Gel-DOC system (Fujifilm, Tokyo, Japan).
SDS-PAGE and Western blot analysis
After drug treatment, nasal tissues were cut into small pieces with a sterile surgical blade and homogenized in liquid nitrogen. Homogenized products were lysed in RIPA buffer (Elpis Biotech, Daejeon, Korea) containing a protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA). To address phosphorylation events, an additional set of phosphatase inhibitors was added to the RIPA buffer (Cocktail II, Sigma-Aldrich). Protein concentration was determined by BCA assay kit (Pierce, Rockford, IL, USA). The proteins (10 µg/sample) were immediately heated for 5 minutes at 100℃. Total cell lysates (5×106 cells/sample) were subjected to SDS-PAGE on gel containing 15% (w/v) acrylamide under reducing conditions. Separated proteins were transferred to nitrocellulose membranes (Millipore Co., Billerica, MA, USA), and then the membranes were blocked with 5% skim milk and commercial Western blot analysis was performed. Chemiluminescence was detected using and ECL kit (Amersham Life Science, Buckinghamshire, UK) and the multiple Gel DOC system (Fujifilm). The following primary antibodies were used: caspase-8, caspase-3, caspase-9, β-actin, Bid, Bax, Bcl-2, Bcl-XL, phospho-Bad (Ser112), Bad, phospho-JNK (Thr183/Tyr185), JNK, phospho-extracellular signal-regulated kinase (ERK)1/2 (Thr202/Tyr204), ERK1/2, phospho-p38 mitogen-activated protein kinase (MAPK) (Thr180/Tyr182), p38 MAPK, phospho-Akt (Ser473), and Akt from Cell Signaling Technology (Beverly, MA, USA). FasL from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and poly(ADP-ribose) polymerase (PARP) from Calbiochem (La Jolla, CA, USA) were used. Data were analyzed using ImageJ 1.38 software (NIH, Bethesda, MD, USA).
Statistical analysis
For the quantifying of nasal polyp apoptosis induced by DEX, densities of FasL, Bcl-2, Bax, and Bad on RT-PCR and proteins on Western blotting were statistically analyzed. The data are presented as mean±SD. The statistical significance of differences between groups was evaluated by the Kruskal-Wallis test with Scheffe for post hoc test (SPSS ver. 17.0, SPSS Inc., Chicago, IL, USA). The level of significance was P<0.05.
RESULTS
FasL expression in nasal polyp induced by DEX treatment
RT-PCR results for mRNA of FasL showed a significant higher expression in DEX-treated polyps than in non-treated polyps in a dose-dependent manner (P<0.01) (Fig. 1). The Western blot analysis also showed that FasL expression was significantly high in DEX-treated polyps than in non-treated polyps in a dose-dependent manner (P<0.01) (Fig. 2).
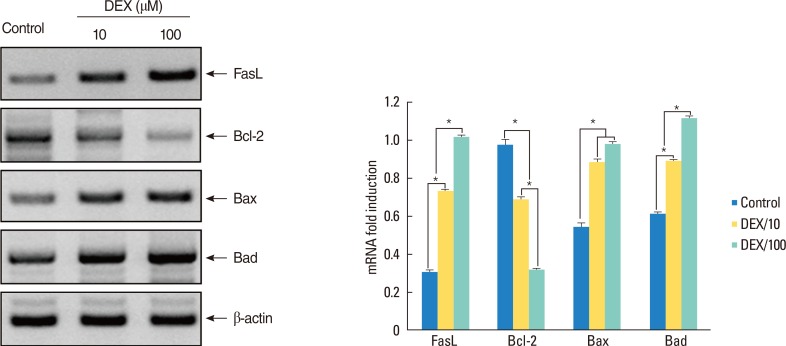
Reverse transcription-polymerase chain reaction analysis for mRNA of FasL and Bcl-2 family molecules. The bars indicate the mean±SD. DEX, dexamethasone. *P<0.01.
Bcl-2 family expression in nasal polyp induced by DEX treatment
RT-PCR showed the expression ratios of pro-apoptotic molecules, Bax (in a dose-independent manner) and Bad (in a dose-dependent manner) increased, but the expression ratio of anti-apoptotic molecule, Bcl-2 decreased in DEX-treated polyps in a dose-dependent manner (P<0.01) (Fig. 1). In the Western blot analysis, expression ratio of pro-apoptotic Bax protein was significantly higher in DEX-treated polyps than in non-treated polyps in a dose-independent manner (P<0.01). However expression ratios of anti-apoptotic Bcl-2 protein (in a dose-dependent manner) and Bcl-XL protein (in a dose-independent manner) were significantly lower in DEX-treated polyps than in non-treated polyps (P<0.01). Expression ratios of activated forms, tBid and p-Bad protein were significantly higher in DEX-treated polyps than in non-treated polyps in a dose-dependent manner (P<0.01). However Bid protein was significantly lower in DEX-treated polyps than in non-treated polyps in a dose-dependent manner (P<0.01) and Bad protein was significantly lower in 100 µM DEX-treated polyps than other polyps (P<0.01), and there was no difference between 10 µM DEX-treated polyps and non-treated polyps (Fig. 2).
Caspases and PARP participate in DEX-induced apoptosis in nasal polyp
In the Western blot analysis, expression ratio of procaspase-8 and procaspase-3 were significantly lower in DEX-treated polyps than in non-treated polyps in a dose-dependent manner (P<0.01), whereas expression ratio of procaspase-9 was significantly higher in DEX-treated polyps than in non-treated polyps in a dose-independent manner (P<0.05). Expression ratios of active forms of caspase-8, caspase-9 (in a dose-dependent manner) and caspase-3 (in a dose-independent manner) were significantly higher in DEX-treated polyps than in non-treated polyps (P<0.01). Expression ratio of PARP was significantly lower, but expression ratio of cleaved form of PARP was significantly higher in DEX-treated polyps than in non-treated polyps in a dose-dependent manner (P<0.01) (Fig. 3).
Akt and MAPKs expression in nasal polyp induced by DEX treatment
In the Western blot analysis, expression ratio of phospho-Akt (in a dose-dependent manner) and phospho-ERK1/2 (in a dose-independent manner) were lower in DEX-treated polyps than in non-treated polyps (P<0.01). Expression ratio of Akt protein was significantly higher in 100 µM DEX-treated polyps than in 10 µM DEX-treated polyps and non-treated polyps and ERK1/2 increased in DEX-treated polyps than in non-treated polyps in a dose-dependent manner (P<0.01) (Fig. 4).
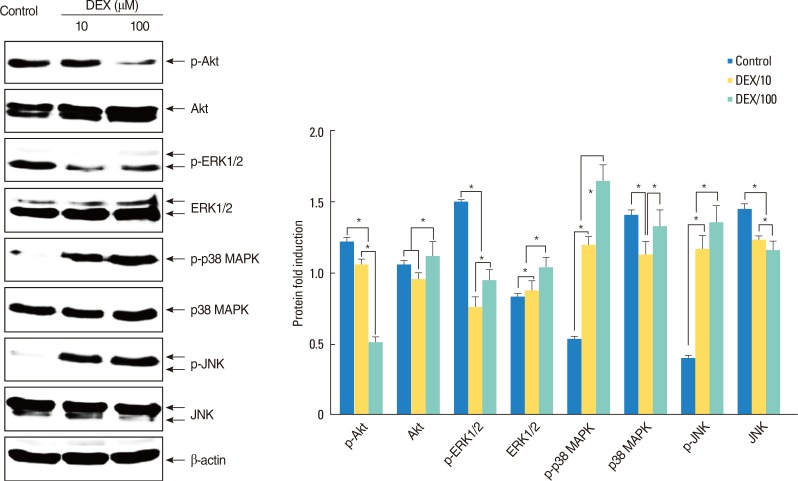
Western blot analysis for Akt, extracellular signal-regulated kinase, p38 mitogen-activated protein kinase, and c-Jun N-terminal kinase. The bars indicate the mean±SD. *P<0.01.
However, expression ratios of phospho-JNK and phosphop38 MAPK were significantly higher in DEX-treated polyps than in non-treated polyps in a dose-dependent manner (P<0.01). JNK decreased in DEX-treated polyps than in non-treated polyps in a dose-dependent manner (P<0.01). There was no difference between polyps in p38 MAPK (Fig. 4).
DISCUSSION
The mechanism of glucocorticoids induced apoptosis performed in lymphoid cells, like thymocytes, has been identified through in vitro studies. The extrinsic apoptotic pathways, such as tumor necrosis factor (TNF), Fas and TRAIL pathway are best characterized for induction of apoptosis. The death-inducing signaling complex (DISC) was first described in the Fas-FasL signaling pathway [10]. Fas-associated death domain contains a death effecter domain that allows binding of procaspase 8, also known as FLICE [11]. After the autoproteolytic cleavage, caspase-8 is released from the DISC [12]. There are two types of cells according to the requirement for mitochondrial pathway in the Fas apoptotic pathway. In type I cells, caspase-8 activates directly other caspases such as caspase-3, and they lead to the execution phase of apoptosis. In type II cells, caspase-8 triggers cleavage of Bid and subsequent release of mitochondrial pro-apoptotic factors such as cytochrome c to drive the formation of apoptosome which is consisted with cytochrome c, apoptotic protease activating factor-1 (Apaf-1), and procaspase-9 [13]. The apoptosome recruits and activates the executioner caspase-3.
PARP is a DNA repair enzyme activated by various environmental stimuli, including free radicals, reactive oxygen species and peroxynitrite [14]. During apoptosis, PARP-1 can be cleaved into 24 and 89 kDa fragments by caspase-3 and caspase-7. The over expression of the 24 kDa fragment inhibits DNA repair and ADP-ribose formation, and stimulates apoptosis [15].
While the initiation and execution of apoptosis depends on activation of the death receptor and/or mitochondrial pathways, many other signaling pathways including PI-3K/AKT, MAPK/JNK, Myc, and NF-κB pathway affect in apoptosis regulation. Akt is an anti-apoptotic signaling molecule, induced by PI3K activation in various growth factor receptor-mediated signaling cascades [16]. MAPKs are important in controlling cellular responses to the environment and in regulating gene expression, mitosis, proliferation, movement, metabolism and apoptosis. Conventional MAPKs are consist of three family members; ERK, JNK, and p38 MAPK. MAPKs are activated by phosphorylation. It has been proposed that activation of JNK and p38 MAPK and inhibition of ERK triggers apoptosis. In the MAPKs, JNK activation correlates with Fas-mediated apoptosis. Daxx, binding protein to the Fas DISC, has been shown to link Fas to the JNK pathways, which may mediate apoptosis through an alternative pathway [17]. In most cases, p38 MAPK pathways are simultaneously activated with JNK pathways.
There are a few studies to determine the effect of glucocorticoids on apoptosis in nasal polyps [4,5,6]. However, these studies only identified the induction of apoptosis in the stromal cells, fibroblasts, eosinophils and T lymphocytes in nasal polyps by glucocorticoids in vitro. Organ cultures preserve the integrity of the cells and matrices in an organ-specific structure and are more likely to be clinically relevant [18]. The response of a whole integrated tissue to a simple ubiquitous signal will reflect not just the specificity of the signal or the receptor affinity, but also the microenvironment of juxtaposed cell types and extracellular matrix [19]. For that reason, authors tried to find any differences of apoptotic pathways between in vitro model of lymphoid cells and ex vivo model of nasal polyps.
In this study, apoptotic pathways, induced by DEX in nasal polyps, were confirmed. However, there are no significant differences compared with the known apoptotic pathways of in vitro models of the lymphoid cells. Recently, a study showed Bax-gene transfer enhanced apoptosis by steroid treatment [20]. In the future, gene therapy will clinically be used as an effective and safe treatment method for nasal polyps. This study has significance in that the glucocorticoids apoptosis pathway in nasal polyps should be confirmed as a basic knowledge for gene therapy. Further study is needed to determine which pathways are dominant.
Notes
No potential conflict of interest relevant to this article was reported.