Preoperative Subclinical Hyperthyroidism in Patients With Papillary Thyroid Carcinoma
Article information
Abstract
Objectives
Numerous studies have reported the effects of subclinical hyperthyroidism on the cardiovascular system, osteoporosis, and metabolic syndrome. However, there are few studies examining the relationships between subclinical hyperthyroidism and thyroid cancer. The aim of this study was to investigate the relationships between preoperative subclinical hyperthyroidism and clinicopathological characteristics in patients with papillary thyroid carcinoma (PTC) in terms of thyroid-stimulating hormone (TSH) levels and TSH receptor antibody (TRAb) values.
Methods
Between January 2001 and December 2007, 462 patients were eligible for analysis in our study; we compared the clinicopathological characteristics of 39 preoperative subclinical hyperthyroidism patients with those of 423 euthyroid patients.
Results
There were no statistical differences between the 2 groups with respect to age, male to female ratio, primary tumor size, extrathyroidal extension (ETE), multifocality, lymph node metastasis, TNM and AMES stages, recurrence, and survival, despite significant difference in TSH concentrations between the 2 groups. In the evaluation for TRAb, primary tumor size was significantly larger in patients with normal TRAb than in patients with elevated TRAb. When the patients were subdivided into 4 categories according to TRAb values (<5.0%; 5.0%-10.0%; 10.1%-15.0%; >15.0%), tumor size and ETE were significantly different. However, we could not find linear relationships in the increase or decrease of TRAb values.
Conclusion
The results of our study suggest that subclinical hyperthyroidism is not independently associated with tumor aggressiveness and prognosis in PTC in spite of reduced TSH levels and increased TRAb values as compared with euthyroid patients.
INTRODUCTION
Subclinical hyperthyroidism is defined by low or suppressed thyroid-stimulating hormone (TSH) with free T4 and T3 concentration within the reference range [1]. Recently, numerous studies have reported the effects of subclinical hyperthyroidism on the cardiovascular system, osteoporosis, and metabolic syndrome [2]. However, there have been few studies examining the relationships between subclinical hyperthyroidism and thyroid cancer, and in the study of thyroid cancer, there have been conflicting and varied results with respect to the roles of thyroid hormones (T4 and T3) and TSH [3]. One set of studies has suggested that TSH can stimulate the development of thyroid malignancy, and that elevated serum TSH levels are also associated with a higher incidence of thyroid cancer and advanced tumor stage [4,5]. In contrast, some have suggested that clinical hyperthyroidism might be associated with aggressiveness of tumors, because thyroid hormone can act as a tumor growth factor mediated by integrin αvβ3 in solid tumors, including thyroid cancer [6,7,8]. In fact, 10-year survival has been reported even in anaplastic thyroid carcinoma with uncorrected hypothyroidism [6]. Although each opinion could be reasonable theoretically, it may also be contradictory. If elevated TSH was independently associated with tumor growth and aggressiveness, then low TSH in hyperthyroidism could be associated with tumor suppression and better prognostic parameters. However, Graves' disease showing over hyperthyroidism is supposed to be associated with tumorigenesis and tumor aggressiveness, despite low TSH concentrations. Some investigators have suggested that TSH receptor antibody (TRAb) is associated with these phenomena [9,10].
The aim of this study was to investigate relationships between preoperative subclinical hyperthyroidism and clinicopathological characteristics in patients with papillary thyroid carcinoma (PTC). Thus, we tried to evaluate whether thyroid cancer behaves aggressively in patients with preoperative subclinical hyperthyroidism, as is seen in overt hyperthyroidism of Graves' disease, or whether the low TSH levels of subclinical hyperthyroidism are associated with better prognostic parameters. Additionally, clinicopathological characteristics according to TRAb values were assessed to identify the effects of TRAb on PTC.
MATERIALS AND METHODS
Patients
Between January 2002 and December 2007, 1,087 thyroid surgeries were performed at our institution. Of these, patients who met the following inclusion criteria were retrospectively evaluated in our study: (1) thyroid surgery performed as initial treatment; (2) histologically confirmed PTC; (3) accessible medical records with preoperative results for thyroid function tests including serum TSH, free T4, and T3 levels; (4) no history of known thyroid disease and thyroid-related medication including antithyroid drugs or levothyroxine; (5) euthyroid status or subclinical hyperthyroidism in preoperative thyroid function test. Euthyroid status was defined as normal levels of serum TSH, free T4, and T3, and subclinical hyperthyroidism was defined as a decrease in serum TSH level below the reference range, with normal serum free T4 and T3 concentrations [1,2]. Because all patients with established clinical or overt hyperthyroidism have been treated or should be treated prior to surgery, we decided that patients with subclinical thyroid dysfunction were best suited for estimating the effect of preoperative TSH concentration on PTC. In accordance with standard practice in our institution's laboratory, the reference ranges of TSH, free T4, and T3 concentrations were defined as 0.45 to 4.5 mIU/L, 0.8 to 2.0 ng/dL, and 0.6 to 1.9 ng/mL, respectively. All TRAb measurements were performed preoperatively with radioreceptor assay kit provided by RSR Ltd. (Cardiff, UK) and the upper normal reference for the TRAb assay was 10%.
Finally, 462 patients were eligible for analysis in our study and we compared the clinicopathological characteristics of 39 patients with subclinical hyperthyroidism with those of 423 euthyroid patients. The cohort of 462 patients enrolled in our study comprised 78 men and 384 women, with a mean age of 46.9 years at the time of diagnosis of PTC. Less than total thyroidectomy was performed in 141 patients, while total thyroidectomy with or without neck dissection was performed in 321 patients. Total thyroidectomy with central neck dissection and without lateral compartment neck dissection was performed in 50 patients. Total thyroidectomy with comprehensive neck dissection including central neck dissection such as radical neck dissection, modified radical neck dissection, and selective neck dissection of levels II to VI was performed in 34 patients, with 3 patients undergoing bilateral neck dissection.
Review of histopathological parameters
The histopathological parameters of each patient were assessed by 1 thyroid pathologist. All pathological slides were reviewed for primary tumor size, presence of multifocal disease, extrathyroidal extension (ETE), and cervical lymph node metastasis. Additionally, because recent studies suggest that Hashimoto's thyroiditis increases the risk for PTC occurrence while playing a role as a positive prognostic indicator, the existence of concurrent Hashimoto's thyroiditis with PTC was also evaluated [11,12]. Pathologic staging was redefined according to the tumor, lymph node, and metastasis (TNM) staging system of the 6th edition of the International Union Against Cancer and the American Joint Committee on Cancer (UICC/AJCC). In addition, the well-established prognosis classification system of differentiated thyroid cancer, the age, metastases, extent, and size (AMES) stage, was used. The patients were staged according to AMES stage into low risk and high risk [13].
Treatment protocol for PTC
All patients underwent surgery performed by the 2 experienced head and neck surgeons. Thyroid lobectomy with isthmusectomy was performed only when the cancer was a unifocal intrathyroidal microcarcinoma (<1 cm in diameter) with no cervical lymph node involvement. In the other cases, total thyroidectomy was primarily performed. Central neck dissection was performed when enlarged lymph node was seen in preoperative imaging studies or in the surgical field of vision with 2.5× loupes, or was detected by palpation of the central neck region. When metastasis to the lateral compartment of the neck was identified in the preoperative evaluation or in follow-up examination after initial thyroid surgery, comprehensive neck dissection was performed from levels II to VI. Radioactive iodine remnant ablation was performed using 100-200 mCi 131I with the same indications as those for total thyroidectomy.
Statistical analyses
SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA) was used to analyse the data. Continuous data are represented as mean±SD. To compare continuous variables, age, tumor size, total follow-up period, concentrations of TSH, free T4, T3, and values of TRAb according to thyroid function status were tested using an independent t-test. The association between subclinical hyperthyroidism or TRAb values (normal vs. elevated) and various clinicopathological factors was assessed using chi-square test or Fisher exact test for age ≥45 years, gender, primary tumor size >1-cm diameter, ETE, multifocality, lymph node metastasis, concurrent HT, subtype of PTC, and stage. We further subdivided the patients into 4 categories according to TRAb values (<5.0%; 5.0%-10.0%; 10.1%-15.0%; >15%) and examined clinicopathological differences among the subgroups using analysis of variance (ANOVA) test for continuous variables and chi-square test for categorical variables. Correlation analysis was performed to evaluate whether a linear relationship existed between TRAb values and continuous variables such as age and tumor size. For categorical variables such as ETE, multifocality, lymph node metastasis, and recurrence, P-value for trend was used to assess the linear relationship with TRAb. With regard to the results, P-values were 2-sided throughout and statistical significance was defined as P<0.05.
RESULTS
Preoperative thyroid function tests
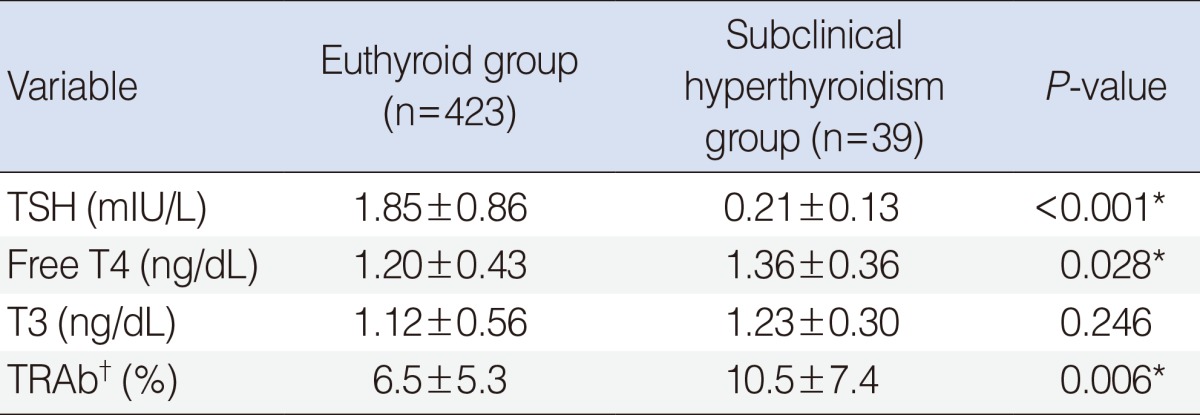
Results of preoperative thyroid function tests of 423 euthyroid patients and 39 patients with subclinical hyperthyroidism are listed in Table 1. The mean concentration of TSH was 1.85 mIU/L in the euthyroid group and 0.21 mIU/L in the subclinical hyperthyroidism group (P<0.001). The mean concentrations of free T4 was significantly higher in the subclinical hyperthyroidism patients than in the euthyroid patients (1.20 ng/dL vs. 1.36 ng/dL, P=0.028), but both were within normal range. There were no differences in the mean T3 concentrations between the 2 groups (1.12 ng/dL in the euthyroid group vs. 1.23 ng/dL in the subclinical hyperthyroidism group, P=0.246).
Among all included patients, preoperative TRAb values were available in 102 patients from the euthyroid group and 19 patients from the subclinical hyperthyroidism group. The mean value of TRAb was 6.5% in the euthyroid patients and 10.5% in the subclinical hyperthyroidism group. This difference was statistically significant, with P=0.006. The proportion of patients with elevated TRAb (>10%) was also significantly higher in the subclinical hyperthyroidism group than in euthyroid patients (46.2% vs. 21.3%, P<0.001).
Clinicopathological characteristics and staging
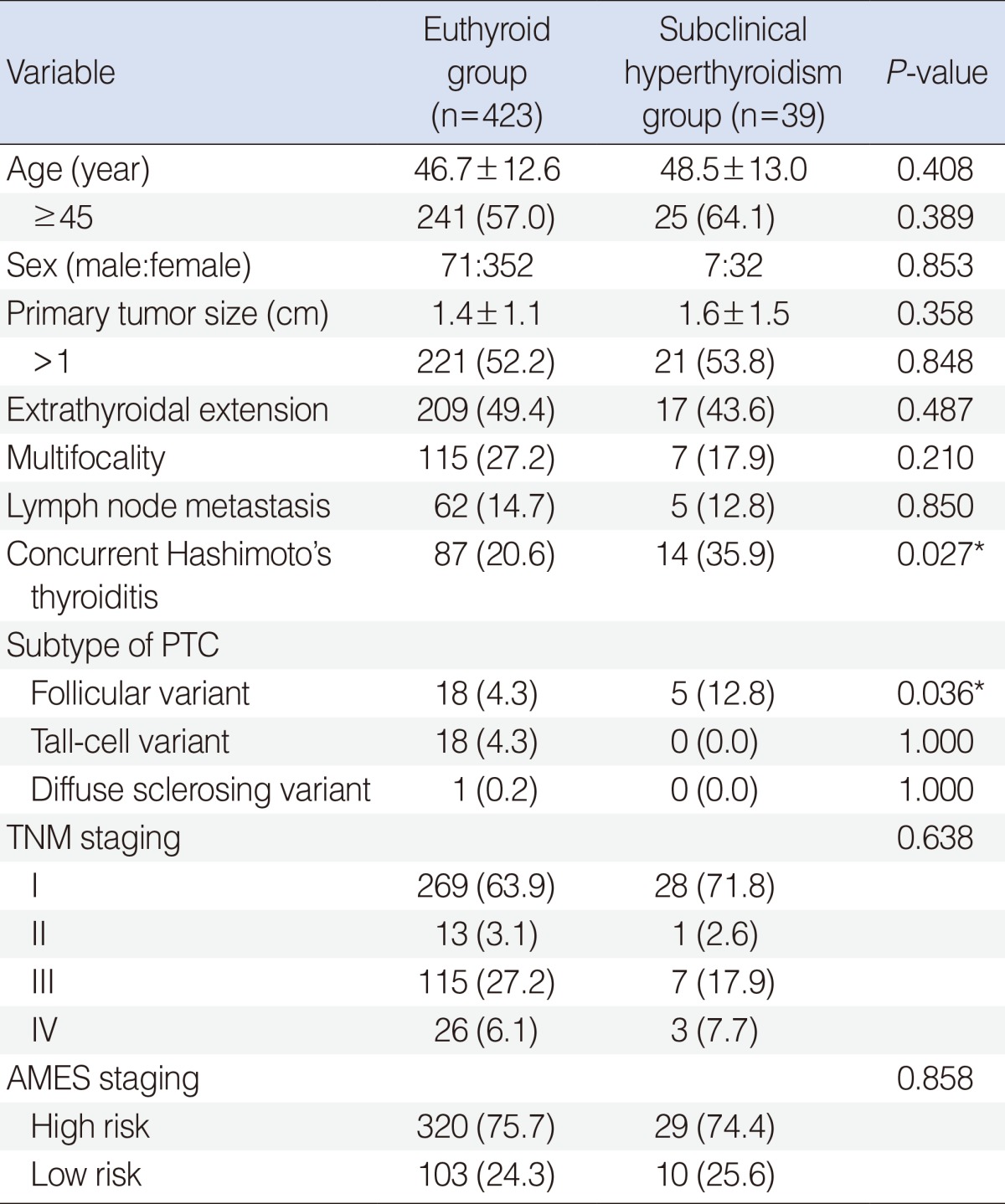
Table 2 lists the clinicopathologial features stratified by euthyroid status or subclinical hyperthyroidism in patients with PTC. There were no statistical differences between the 2 groups with respect to age (46.7 years vs. 48.5 years, P=0.408), male to female ratio (71:352 vs. 7:32, P=0.853), primary tumor size (1.4 cm vs. 1.6 cm, P=0.358), ETE (49.4% vs. 43.6%, P=0.487), multifocality (27.2% vs. 17.9%, P=0.210), and lymph node metastasis at the time of initial diagnosis (14.7% vs. 12.8%, P=0.850). Concurrent Hashimoto's thyroiditis was more frequently found in the subclinical hyperthyroidism group than in the euthyroid group (35.9% vs. 20.6%, P=0.027). As to subtype of PTC, the proportion of follicular variant PTC (FVPTC) was significantly higher in the subclinical hyperthyroidism group than in the euthyroid group (12.8% vs. 4.3%, P=0.036). Evaluation of the expectant prognostic outcome using the TNM and AMES staging systems showed no differences between 2 groups with regard to the proportion at each stage (P=0.638) and the proportion of high risk patients (P=0.858) (Table 2).
Recurrence and survival as a function as thyroid hormone state
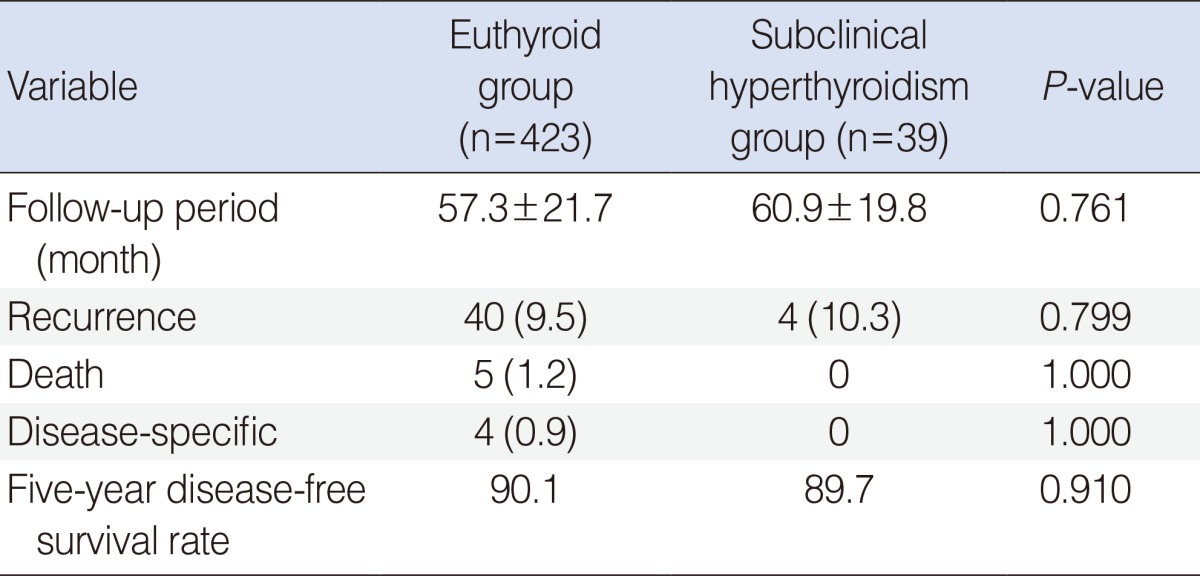
During a 57.3-month follow-up period in the euthyroid group and a 60.9-month follow-up in the subclinical hyperthyroidism group, recurrence occurred in 40 of 423 euthyroid patients (9.5%) and 4 of 39 patients (10.3%) with subclinical hyperthyroidism. This difference was not statistically significant (P=0.779). Overall, 5 patients of the euthyroid group (1.2%) died, and 4 of these deaths (0.9%) were due to PTC or related complications. All patients of the subclinical hyperthyroidism group were alive at the time of the present evaluation. The 5-year disease-free survival rates were 90.1% and 89.7% in the euthyroid group and the subclinical hyperthyroidism group, respectively (P=0.910) (Table 3).
Prognostic factors according to TRAb value
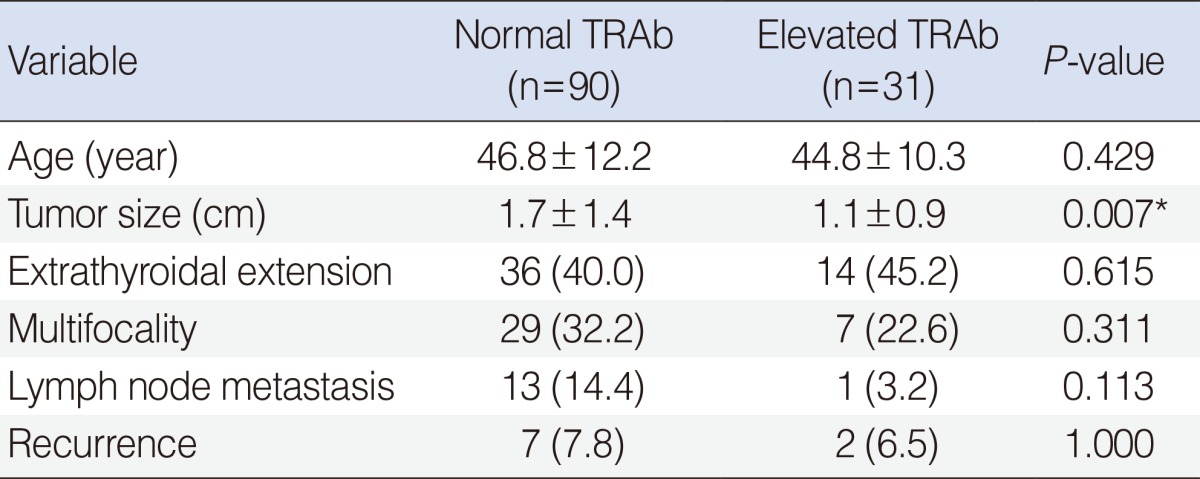
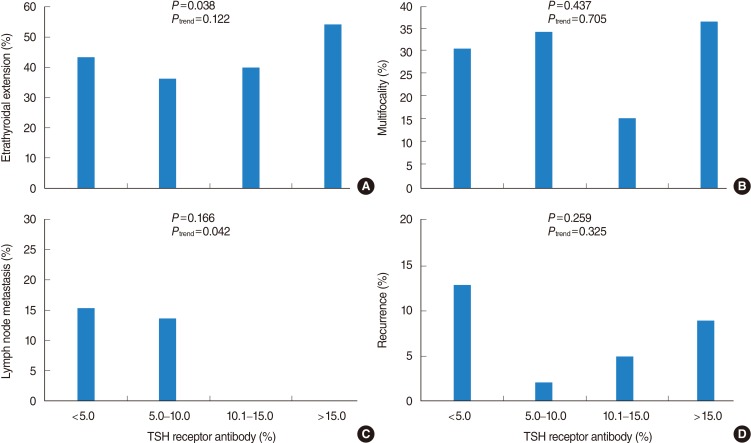
In the evaluation of several prognostic parameters including age, tumor size, ETE, multifocality, lymph node metastasis, and recurrence according to the TRAb value (normal or elevated), primary tumor size was significantly larger in patients with normal TRAb value than in patients with elevated TRAb (1.7 cm vs. 1.1 cm, P=0.007) (Table 4). However, other parameters had no significant differences according to the normal or elevated TRAb values. When the patients were subdivided into 4 categories according to TRAb values (<5.0%; 5.0%-10.0%; 10.1%-15.0%; >15.0%), tumor size and ETE were significantly different among the 4 categories, with P=0.024 and P=0.038, respectively. Pearson correlation coefficients (r) for age and tumor size to evaluate for any positive or negative relationship corresponding to an increase in TRAb value were r=-0.093 and r=-0.035, respectively, indicating that there were negligible linear relationships (Fig. 1A, B). The P-values for trend in ETE, multifocality, and recurrence were 0.122, 0.705, and 0.325, respectively (Fig. 2A, B, D). With respect to lymph node metastasis, although P for trend was 0.042, there was no significant difference among the 4 categories of TRAb, with P=0.166 (Fig. 2C).
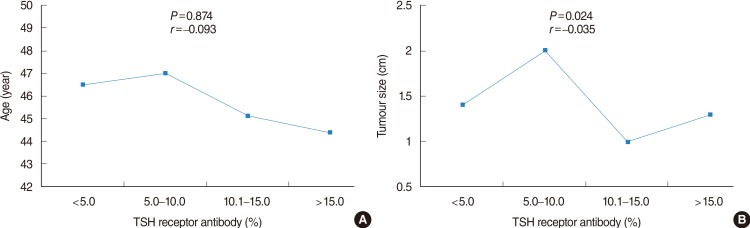
Mean age (A) and tumor size (B) according to preoperative thyroid-stimulating hormone (TSH) receptor antibody values. r=Pearson correlation coefficient.
DISCUSSION
Recently, several studies have reported clinical relationships between TSH and thyroid cancer [4,5,14]. A study evaluating 554 patients with well-differentiated thyroid cancer found that TSH ≥2.5 mIU/L was associated with a higher incidence of ETE and lateral lymph node metastasis [4]. In a study of 10,178 patients who underwent fine needle aspiration, lower TSH levels were associated with a lower risk of papillary thyroid cancer [5]. However, these studies did not take thyroid hormone (T4 and T3) concentrations into consideration, despite the fact that elevated TSH usually denotes decreased thyroid function, and low levels of TSH usually denote increased thyroid function, leaving it unclear whether the effect of elevated/decreased TSH on malignant tumors ensues from decreased/increased thyroid hormones or the TSH itself. Therefore, we proposed that it would be more reasonable to consider thyroid function status rather than TSH level alone to evaluate the relationship of TSH and thyroid cancer. For this purpose, we restricted our analysis to subclinical hyperthyroidism with normal free T4 and T3 concentrations, thus eliminating bias introduced by effects of endogenous or exogenous thyroid hormone, and making it possible to compare the effects of low TSH levels in subclinical hyperthyroidism patients with PTC directly to the effects of normal TSH levels in euthyroid PTC patients.
We found that there was no difference in prognostic parameters including age, sex, tumor size, ETE, multifocality, and lymph node metastasis between the euthyroid and subclinical hyperthyroidism groups despite of significant difference of TSH concentrations between the 2 groups. This result suggested that TSH is not an independent factor which was associated with tumorigenesis or metastatic potential. In fact, TSH was not elevated in patients with micro-PTC in a recent study, indicating that is not likely involved in the de novo oncogenesis of PTC [14]. Moreover, several recent in vitro studies have suggested that non-genomic actions of thyroid hormone mediated by integrin αvβ3 were responsible for angiogenesis in tumors and that T4-αvβ3 complex acted by activating a mitogen-activated protein kinase (MAPK) dependent signalling pathway as a growth factor in papillary and follicular thyroid cells [15,16]. Therefore, even if low levels of TSH in subclinical hyperthyroidism were associated with a suppressive effect on PTC relative to a euthyroid state, it is possible that higher level of T4 will act as a stimulating factor of PTC mediated by integrin αvβ3 in both clinical and subclinical hyperthyroidism. With respect to subclinical hypothyroidism, we have already reported the results of a study that compared the characteristics of PTC in patients with subclinical hypothyroidism (elevated TSH with normal thyroid hormones) with those in patients with euthyroid status (normal TSH with normal thyroid hormones), and we found no significant differences for several prognostic parameters according to the TSH levels [17]. Therefore, we consider that our 2 studies show coherent results indicating that preoperative TSH levels have no clinical impact on the characteristics of PTC, as long as thyroid hormone levels (T4 and T3) are within normal ranges.
In our study, patients with subclinical hyperthyroidism had greater proportion of FVPTC compared with patients with the euthyroid state. FVPTC has been regarded as a more aggressive form of thyroid cancer that warrants intensive therapy [18]. However, it is difficult to conclude that subclinical hyperthyroidism was associated with poor prognostic parameters due to the low incidence FVPTC (12.8%, even in subclinical hyperthyroidism). In fact, with respect to TNM and AMES staging, both groups had a similar proportion of patients in each stage and high-risk group, and there was no difference in recurrence and survival between the 2 groups during follow-up period of 5 years.
Of the total 462 patients, a preoperative TRAb performed by a radioreceptor assay kit was available in 121 patients. In the present study, all TRAb measurements were. In this method, 125I-labeled bovine TSH competes with TRAb in serum samples to bind to purified porcine TSH receptors, followed by polyethylene glycol precipitation [19,20]. In our institution, new generations of TRAb assays have been used since 2009, and it is known that a minor subset of sera from patients with Graves' disease are falsely negative using the assay employed in our study [20,21].
Some investigators have found that multifocality and distant metastasis of thyroid cancer were more frequent in Graves' disease patients than in euthyroid patients [9,10]. In addition, high serum levels of TRAb in Graves' disease have been reported to stimulate the growth of thyroid cancer [22]. In our study, patients with subclinical hyperthyroidism had significantly higher mean values of TRAb compared with euthyroid patients (10.5% vs. 6.5%, P=0.006) and elevated TRAb (>10%) was also identified more frequently in the subclinical hyperthyroidism group than in the euthyroid group (46.2% vs. 21.3%, P<0.001). However, we could not find any adverse tumor characteristics in patients with subclinical hyperthyroidism as supported in PTC of Graves' disease compared with euthyroid patients [23]. Rather, the mean tumor size was smaller in the subclinical hyperthyroidism group than in the euthyroid group (1.1 cm vs. 1.7 cm, P=0.007). Although tumor size and ETE were significantly different (P=0.024 and 0.038, respectively) among 4 categories stratified according to TRAb values, there were no linear relationships as TRAb increased or decreased. Interestingly, mean tumor size was largest in patients within the 5.0%-10% TRAb subgroup and smallest in patients within the 10.1%-15% TRAb subgroup. In addition, although there were no differences among the 4 categories in lymph node metastasis at initial diagnosis, there were negative linear relationships as TRAb values increased. These results did not correspond to the conventional hypothesis that TRAb plays a role in growth and aggressiveness of thyroid cancers [22,24]. In a recent study evaluating the clinicopathological characteristics and prognosis of PTC in Graves' disease patients and euthyroid patients, tumor size was smaller in Graves' disease patients although it was not significant [23]. Furthermore, there was an inverse correlation between the TRAb values and tumor size, and ETE and lymph node metastasis were not associated with TRAb values, suggesting that the absolute role of TRAb on tumorigenesis and tumor aggressiveness is limited [23].
In the present study, concurrent Hashimoto's thyroiditis was more frequently found in subclinical hyperthyroidism patients than in euthyroid patients. Interestingly, most studies investigating the clinical relationship between Hashimoto's thyroiditis and PTC report a better prognosis for PTC concurrent with Hashimoto's thyroiditis compared with PTC alone [11,12]. Hashimoto's thyroiditis is a kind of autoimmune disease that leads to the destruction of thyroid follicles through an immune response to a thyroid specific antigen. As PTC cells originating from the follicular cells would express the thyroid specific antigen, autoantibodies from coexisting Hashimoto's thyroiditis might destroy the tumor cells in much the same way as in Hashimoto's thyroiditis alone [11]. Additionally, the infiltrated lymphocytes in patients with PTC are likely to be cytotoxic T cells acting as carcinoma cell killers, secreting interleukin-1 that inhibits thyroid cancer cell growth [12,25]. Therefore, even though the significant higher values of TRAb in the subclinical hyperthyroidism group, compared with the euthyroid group, may affect the clinicopathological characteristics of PTC, the significantly higher incidence of concurrent Hashimoto's thyroiditis in the subclinical hyperthyroidism group may have compensated for this effect in our study population. This interplay may have led to the final observed result: there were no significant differences between the 2 groups for the several prognostic parameters.
Our study has limitations including the small sample size of subclinical hyperthyroidism patients and insufficient follow-up duration. Because the overall prognosis of PTC is remarkably excellent, it is difficult to analyze recurrent and survival differences between subgroups of PTC [12]. However, we deemed that a mean 59-month of follow-up is not too short-term as a period to evaluate recurrence, although it is not sufficient to evaluate survival. Another weakness of the present study is that we could not directly evaluate whether low TSH in subclinical hyperthyroidism was associated with the decreased incidence of PTC because the study was based on patients undergoing thyroid surgery as treatment for this malignancy. In addition, although it was within the normal range, subclinical hyperthyroidism patients had higher concentrations of free T4 than did euthyroid patients. Despite these limitations, the results of our study suggest that subclinical hyperthyroidism is not independently associated with tumor aggressiveness and prognosis in PTC in spite of the reduced TSH levels and increased TRAb values present in subclinical hyperthyroidism. In the future, long-term prospective cohort studies of the general population with/without PTC should be performed with the hopes of better understanding the significance of TSH and TRAb values in relation to the occurrence and characteristics of PTC.
Notes
No potential conflict of interest relevant to this article was reported.