Association Study of FOS-Like Antigen-2 Promoter Polymorphisms With Papillary Thyroid Cancer in Korean Population
Article information
Abstract
Objectives
FOS-like antigen-2 (FOSL-2), a member of the FOS gene family, encode leucine zipper proteins that can heterodimerize with proteins of Jun family. Thus, activating protein (AP)-1 transcription factor is formed, has a crucial role in proliferation, differentiation and apoptosis of normal tissue as well as oncogenic transformation and progression. We performed an association study of single nucleotide polymorphisms (SNPs) in the FOSL-2 with papillary thyroid cancer (PTC). We also estimated the relationships between the SNPs and the clinicopathologic characteristics of PTC.
Methods
One promoter SNPs (rs925255) of FOSL-2 gene were genotyped with direct sequencing method in 94 PTC and 213 controls. PTC patients were dichotomized and compared with respect to clinical parameters of PTC. Genetic data were analyzed using Helixtree, SNPAnalyzer, SNPStats. Multivariate logistic regression analysis was fulfilled to evaluate the genetic effect with adjustment for age and sex.
Results
SNP (rs925255) in FOSL-2 showed a significant association (codominant 1 model [G/G vs. A/G]: odds ratio [OR], 0.531, 95% confidence interval [CI], 0.293 to 0.96, P=0.036; dominant model: OR, 0.50, 95% CI, 0.28 to 0.89, P=0.015) with PTC. The frequency of allele G in rs925255 was also significantly associated with PTC (OR, 0.59; 95% CI, 0.34 to 0.91; P=0.02). But we fail to prove significant association between this polymorphism (rs925255) and clinico-pathological parameters.
Conclusion
Our findings suggest that the rs925255 SNP and its allele G show significant association with the PTC in Korean population.
INTRODUCTION
Thyroid cancer is the most common neoplasm in Korea [1]. Recently, its incidence is an explosive increase. In US, approximately 19,500 new patients of thyroid carcinoma (1% of all new case of cancer) are diagnosed each year [2]. The papillary thyroid cancer (PTC) is most common type, approximately 70% to 80% of differentiated thyroid cancer (DTC). Most of the DTC has a good prognosis but diffuse sclerosing, tall and columnar cell variants have more aggressive clinical course [2].
It is well-known that radiation exposure is to the PTC what iodine deficiency is to follicular thyroid cancer (FTC). In the Chernobyl disaster, the incidence of PTC is increased 100- to 200-fold, especially in a child [3]. Several genetic alterations are closely associated with the specific type of the thyroid cancer [4]. PTC has a genetic tendency that is a three-to eight-fold rate of increase in the first-degree relatives, this is the highest of all cancers [5,6]. In spite of such recent progression of knowledge, we have still limited understanding about the molecular and biological characteristics in the thyroid neoplasm.
The FOS gene family is made up four members: c-FOS, FOSB, FOS-like antigen (FOSL) -1 and -2. The Jun family consists of c-Jun, JunB and JunD. The FOS gene encodes leucine zipper proteins that can heterodimerize with Jun family. In this way, activating protein (AP)-1 transcription factor is formed [7]. The FOSL-1 over-expression is associated with hyperplastic and neoplastic thyroid diseases [8]. Generally, the FOSL-2 is believed to be associated with the cell adhesion, motility, invasiveness and metastasis than cell growth [9]. In previous study, it is associated with the tumorigenesis of colorectal, breast cancer and ischemic injury [10]. But the correlation between FOSL-2 and PTC has not been clarified. In the last few years, the association between single nucleotide polymorphisms (SNPs) of gene and thyroid cancer have been reported.
The aim of this study is to trace the association between SNPs of FOSL-2 and risk of PTC. To prove our hypothesis, we performed a hospital-based case-control study in Korean population to compare the frequencies of polymorphisms of the FOSL-2 between PTC patients and control subjects. We also estimated the relationships between FOSL-2 SNPs and the clinico-pathologic characteristics of PTC.
MATERIALS AND METHODS
A hospital-based case-control study was designed to compare the frequencies of polymorphisms of the FOSL-2 between PTC patients and control subjects.
Patients and controls
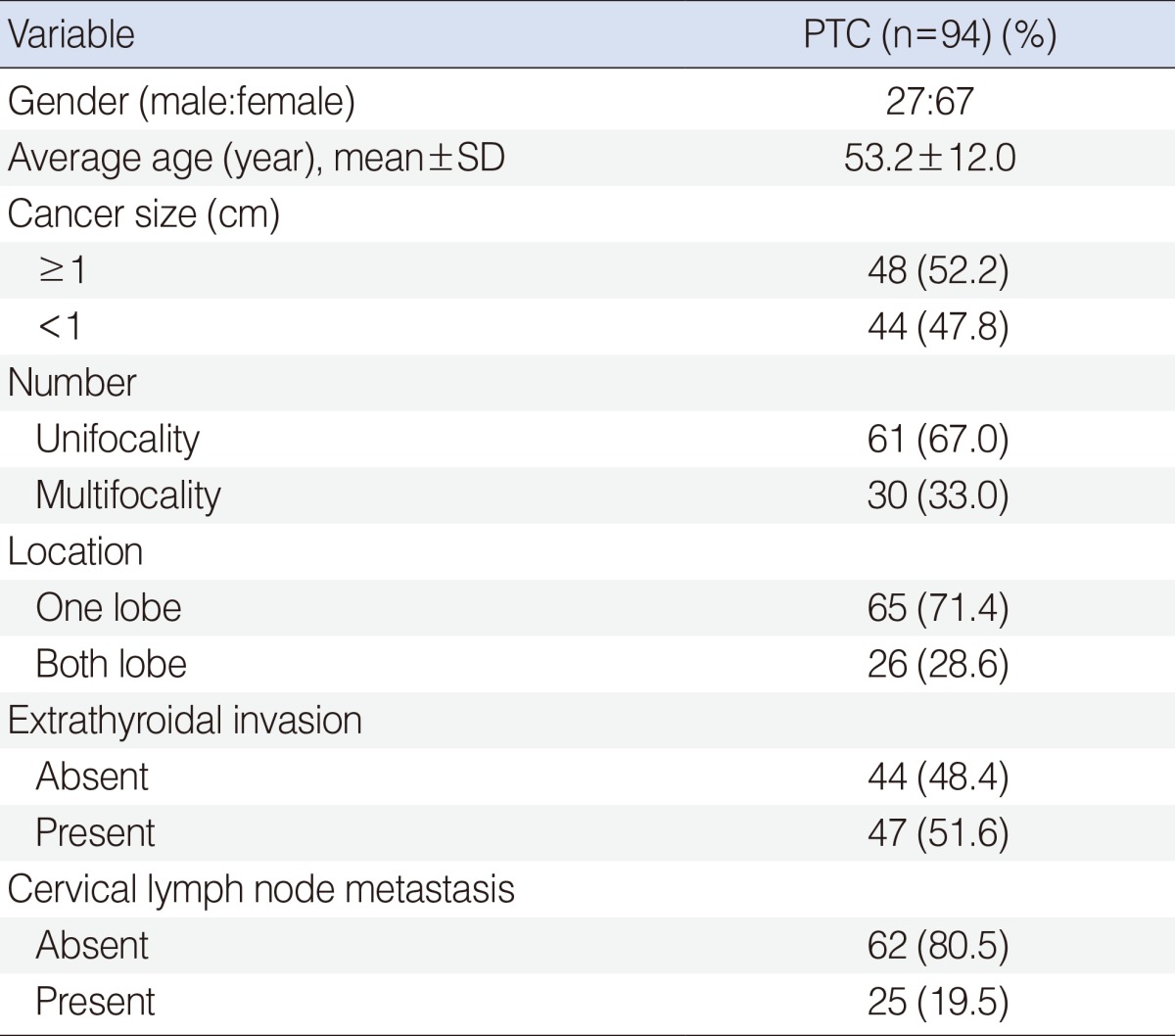
We enrolled 94 patients with PTC and 213 control subjects at Kyung Hee University Medical Center, VHS Medical Center and Ilsong Memorial Institute of Head and Neck Cancer. All of patients with PTC and control subjects are Korean. The diagnosis of PTC and the presence of cervical lymph node metastasis were confirmed by pathologic diagnosis. None of the controls had been diagnosed with cancer or thyroid disease at enrollment. Demographic data of PTC patients and controls are summarized (Table 1).
This study was approved by the institutional review boards of the Medical Research Institute on both hospitals. Written informed consent was obtained from all subjects directly.
Patient subgroups
To decide the nature of the association between FOSL-2 SNP and the clinico-pathologic characteristics of PTC, we divided the patients into subgroups according to size of cancer (<1 cm and ≥1 cm), number of tumor (unifocality and multifocality), location of tumor (one and both lobe), extrathyroidal invasion (+) and (-) group and lymph node metastasis (+) and (-) groups in the pathology. The small differences in subgroup numbers were caused by loss of some clinical data. Age and gender of the control group were matched to those of the PTC group.
SNP selection and genotyping
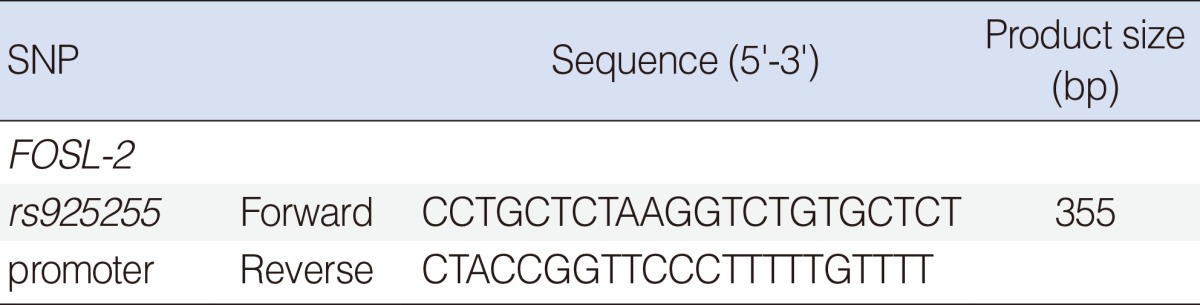
We searched the SNPs of FOSL-2 genes. The connected information of the SNPs was gained from the SNP database (www.ncbi.nlm.nih.gov/SNP, dbSNP Build 131) of the National Center of Biotechnology Information. Among the SNPs of the promoter region (regulatory SNP, rSNP) in FOSL-2, SNPs with below 0.05 heterozygosity (rs75967881), without genotype frequencies (rs11677002, rs72408887, rs5830070, rs34014644, rs5830071, rs34865339, rs4042624, rs1876774) were excluded. The heterozygosity of rs11677002 is 0.500, but no previous Asian data is existed. Finally we selected SNPs rs925255 of FOSL-2. Heterozygosity of this SNP is 0.428 respectively (dbSNP BUILD 131). Genomic DNA was extracted from blood sample collected in Na-EDTA tubes using Roche DNA Extraction kit (Roche, Indianapolis, IN, USA). Polymerase chain reactions (PCRs) were performed using specific primer for the rs925255 (Table 2). PCR products were sequenced using an ABI PRISM 3730XL analyzer (PE Applied Biosystems, Foster City, CA, USA). The Sequence data were analyzed by SeqManII software (DNASTAR Inc., Madison, WI, USA).
Statistical analysis
For all SNPs, compliance with the Hardy-Weinberg equilibrium (HWE) was assessed using SNPstats software (http://bioinfo.iconcologia.net/index.php?module=Snpstats) in patients and controls, and adjusted for age and sex. We analyzed the genetic data, using Helixtree (Golden Helix Inc., Bozeman, MT, USA) and SNPAnalyzer (Istech Inc., Goyang, Korea). Multiple logistic regression models (codominant, dominant, and recessive) were applied to get odds ratio (OR), 95% confidence interval (CI) and P-value. Analysis of data was performed using SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA). Statistical significance was set at P<0.05.
RESULTS
The genotypic distributions of all SNPs in this study were consistent with the Hardy-Weinberg equilibrium (P>0.05, data not shown). SNP (rs925255) in FOSL-2 showed a significant association in codominant1 (G/G vs. A/A) and dominant model with PTC. The frequency of allele G in rs925255 was significantly higher in the PTC patients than controls (Table 3). We assessed the genetic relationships between this SNP and subgroups of PTC patients, no clinical parameter was significantly associated with the SNP of FOSL-2. We calculated the sample power to verify our data (http://www.stat.ubc.ca/~rollin/stats/ssiz e/b2.html). The sample power of the SNP was 0.788 (rs925255, number of cases for 70% power=76). Thus our results were acceptable.
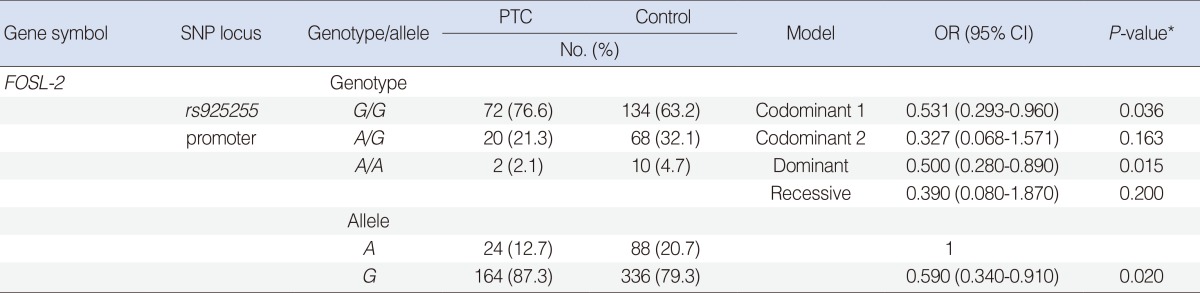
Genotype and allele frequencies of FOSL-2 promoter polymorphisms in papillary thyroid cancer and control subjects
To check the effect of this SNP, we used the Alibaba 2.1 (http://www.gene-regulation.com/pub/program/alibaba2) to detect whether promoter SNPs affect transcription factors. At rs925255 SNP of the FOSL-2 gene, the C-containing sequence binds with GCN4 and Ftz transcription factors. After the change into the T-containing sequence, the binding transcription factor converts into the Antp transcription factors.
DISCUSSION
This study is the first attempt to find out relationship between FOSL-2 and PTC in Korean population. Also, we assessed the relationship between FOSL-2 and clinicopathological characteristics of PTC. The main results of this study are summarized as follows [1]; The genotype frequencies of promoter SNP (rs925255) in FOSL-2 were significantly associated with PTC [2]. The frequency of major allele G in rs925255 was significantly higher in PTC. These findings suggest that SNP rs925255 is closely associated with PTC.
As we mentioned earlier, the role and biology of FOSL-2 in malignant tumor are less well understood than other FOS gene members. The FOSL-2 gene is located in chromosome bands 2p22-p23. The role of FOSL-1 and -2 about tumor progression in vivo remains unclear, but the following mechanisms are relatively well established. In the transformed cell, several oncogenes such as v-src, c-Ha-Ras, activated Raf or MEK1 gene (MEK-DD) cause elevation of endogenous MAPK (mitogen-activated protein kinase) activity. This activity converts FOSL-2 into hyperphosphorylation, formed a FOSL-2/c-Jun heterodimer [11]. It has transcriptional activity, so-called AP-1. This transcription factor plays a pivotal role in the proliferation and differentiation of normal tissue as well as oncogenic transformation and progression. Thus, FOS gene family acts as regulators in cell metabolism and apoptosis [11,12].
Because the PTC has a strong genetic trait and explosive increasing tendency, many studies had been performed to find out the pathogenesis about PTC. Among these studies, the BRAFV600E transversion at position 1799 recently gets the limelight. It is the most common genetic alteration (average 40%) in PTC, plays an important role in carcinogenesis and disease prognosis. The next thing, RET/PTC (rearranged in transformation papillary, rearranged during transformation), Galectin-3 and NTRK 1 (neurotrophic receptor-tyrosine kinase) are well known studied at this time [2,13].
Recently, several research groups conduct the SNP study about thyroid cancer. The -106A/G of interleukin 11 receptor-α promoter polymorphism may be associated with PTC risk [14], the 1513A>C polymorphism in the purinergic receptor P2X7 is strongly associated with the follicular variant of PTC [15]. The polymorphism of IL-6 (G-174 C), IL-10 (-1082 G/A), DNA repair gene (RAD52) and estrogen receptor 1 (rs2228480) show significantly associated with PTC [16-19].
There are millions of SNP in human body. Among these, only a few SNPs are functional role in protein expression. The final goal of such studies is to develop the SNP into the screening method and target therapeutic agent of PTC. In general, promoter SNP (rSNP, regulatory SNP) and missense SNP (cSNP, coding SNP) are great likelihood to be a significant function. The rs925255 in our study is located at the promoter region of FOSL-2 gene. As appears by the result in Alibaba 2.1, this SNP has good possibility to be a functional SNP in expression of FOSL-2. Assuming that promoter SNP affects transcription factor binding, this promoter SNP may influence gene and protein expression of FOSL-2. Finally, It leaves much to be desired that all of the clinical parameters were not significantly associated with PTC.
This study is the first step to find out the relationship between FOSL-2 gene and thyroid cancer. We feel keenly the necessity of a more functional study about thyroid cancer and FOSL-2 gene. Therefore, we will plan to more study in the near future through the Northern blot and ELISA. Thus, we will try to detect the functional differences between the genotypes of SNP, expression patterns of FOSL-2 in normal and pathologic thyroid tissues and association with other types of thyroid cancer. Ultimately, we anticipate that the discovery of SNP in FOSL-2 gene as a marker for PTC could supply a tool to forecast a high-risk patient or could lead to the detection of potential therapeutic targets.
In conclusion, we performed the association study about the promoter SNP (rSNP) of FOSL-2 gene and PTC. The SNP (rs925255) of FOSL-2 showed a significant association with the PTC in Korean population. The frequency of allele G in rs925255 was higher in the PTC. Additional experiments for uniform techniques of detection and large international collaborative studies could clarify the uncertainties about the role of FOSL-2 to PTC. To the best of our knowledge, this is the first report about the association of FOSL-2 SNP and PTC with analyzing its clinical parameters.
Notes
Presented at the 14th International Thyroid Congress, Paris, France, September 11-16, 2010.
No potential conflict of interest relevant to this article was reported.