Endoscopic "Push-Trough" Technique Cartilage Myringoplasty in Anterior Tympanic Membrane Perforations
Article information
Abstract
Objectives
To evaluate endoscopic push-through technique cartilage myringoplasty results.
Methods
This prospective study was performed on patients with anterior tympanic membrane perforations and endoscopic push-through technique cartilage myringoplasty was performed between 2011 and 2013. The patients who did not have any cholesteatoma or otorrhea in the previous 3 months, and had an air bone gap ≤25 dB in their preoperative audiograms were included in the study. They were followed up with endoscopic examination and audiograms at 2nd, 6th, 12th, and 24th postoperative months. Pure tone averages were calculated at 0.5, 1, 2, and 4 kHz frequencies.
Results
Of 32 patients, 19 were females and 13 were males. The mean age was 40.3 years (range, 16 to 62 years), and the mean follow-up period was 12.4 months (range, 6 to 24 months). Graft success rate was 87.5% in this study. Preoperative mean air conduction hearing threshold was 25.9 dB, and the mean air-bone gap was 11.9 dB while these values improved to 19.5 dB and 5.3 dB respectively in the postoperative period. The mean hearing gain was 6.4 dB. The analysis of preoperative and postoperative mean air conduction thresholds and air bone gap values of the patients revealed statistically significant differences.
Conclusion
Underlay cartilage myringoplasty with endoscopic push-through technique in anterior quadrant tympanic membrane perforations is an effective, minimally invasive and feasible method.
INTRODUCTION
Microscopic myringoplasty is one of the most frequently performed traditional procedures. A prominent anterior canal wall, and anterior quadrant or marginal perforations may limit the exposure or surgical manipulations when an operation microscope is used for visualization of the surgical field [1234]. In these cases, in order to overcome the disadvantages of using a microscope, more invasive procedures that enlarge surgical field are needed such as canaloplasty or a postauricular approach [4]. Use of endoscopes provides considerable advantages with a wide exposure.
Repair of tympanic membrane's (TM's) anterior quadrant perforations is harder than the repair of the posterior perforations due to graft's viability, protrusion of the anterior canal wall and medialization of the graft. Graft is usually placed medial to the tympanomeatal flap with an underlay technique for the repair of anterior quadrant perforations.
In this study, endoscopic transcanal push-through technique underlay cartilage myringoplasty was performed in patients with anterior quadrant perforations, some of whom with prominent anterior external acoustic canal (EAC) walls, and the results were analyzed.
MATERIALS AND METHODS
This study is a prospective study aimed to analyze the results of endoscopic transcanal "push-through" technique underlay cartilage myringoplasty (EPTCM) performed in patients with anterior quadrant TM perforations between 2011 and 2013. The inclusion criteria were; presence of an anterior quadrant TM perforation, a dry ear in the previous 3 months, absence of a cholesteatoma, and ≤25 dB air-bone gap (ABG) in the preoperative audiogram. Thirty two patients who fulfilled these criteria and operated by the first senior author were included in the study.
Technique
Surgery was performed under local anesthesia except children and the patients who could not tolerate general anesthesia. The patients who had the operation under local anesthesia had premedication with 10 mg diazepam peroral, and 100 mg meperidine, 50 mg promethazine, and 0.4 mg atropin intramuscular, 2 hours before surgery. Topical anesthesia was performed with 2% lidocaine and 1:100,000 epinephrine injections to posterior, inferior, superior, and anterior walls of the external ear canal.
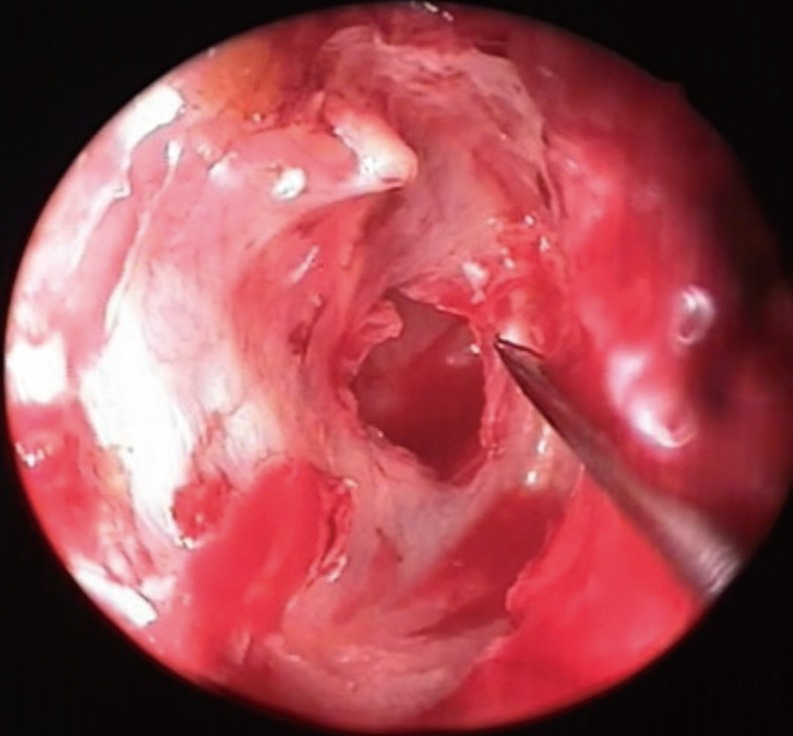
First, perforation edges and anterior annulus were visualized with a 0°, 4-mm×18-cm rigid endoscope. The edges of the perforation were desepitelized with an angled pick (Fig. 1). Later, a 4- to 5-mm incision was performed 2- to 3-mm medial to the tragal cartilage's free border, incising the skin and the cartilage. The cartilage was freed of the perichondrium, and elevated at the EAC aspect of the cartilage. On the opposite side, the perichondrium left attached to the cartilage, and a 3- to 4-mm-sized cartilage was removed with its perichondrium attached on one side, the other side of it being uncovered by perichondrium. The incision was sutured with an absorbable material. The ear canal-side of the cartilage is concave, therefore this side was preferably placed towards the middle ear. Under endoscopic vision, the middle ear was tightly packed with gelfoam through the perforation, until the level of the perforation. The tubal orifice was supported more tightly in order to prevent medialization due to negative pressure produced by sniffing. Later, the size and the diameter of the perforation were measured using a sterile piece of aluminum foil. The foil was placed on the cartilage, and the graft was prepared 1-2 mm larger it. The tragal cartilage was not thinned out, and used as a graft at its natural thickness. The cartilage graft was pushed through the perforation, and placed in an underlay fashion, its concave and perichondrium - free surface looking towards the middle ear (Figs. 2, 3). It was covered with gelfoam until the level of the isthmus.
Follow-up
The patients were discharged on the day of the surgery. The patients were given oral antibiotics and antihistamines in the first postoperative week, as well as nonsteroid anti-inflammatory agents, as needed. The gelfoam in the EAC was removed at postoperative 3rd week, and the status of the graft was evaluated. Then, the patients were followed up at postoperative 2nd, 6th, 12th, and 24th months using endoscopic examination and audiometry.
Analysis
Duration of surgery
Time between start of surgery after general anesthesia induction and the end of surgery was taken into consideration in patients who had surgery under general anesthesia, and the time between the start of local anesthesia and the end of surgery was taken into consideration in patients who had surgery under local anesthesia.
Graft success rate
Graft insufficiency was defined as a gap in perforation due to graft retraction and medialization in postoperative 2nd month. Complication was defined as appearance of epithelial pearls at the edges of the perforation and reperforation due to infection after postoperative 2nd month.
Evaluation of hearing
The audiometric evaluations were done using preoperative (within one month, before surgery) and postoperative (>6 months postoperatively) audiograms. Pure tone averages were calculated at frequencies of 0.5, 1, 2, and 4 kHz. Closure of ABG to 10 dB or smaller was regarded as the success criterion.
Statistical analysis
The analysis of data was performed using SPSS ver. 11.5 (SPSS Inc., Chicago, IL, USA). Frequency was used for variables obtained by counting, and mean±standard deviations (median [range]) were used for the variables obtained by measuring, as the descriptive measures. Wilcoxon signed ranks test was performed for comparison of pre- and post measurements. P<0.05 was regarded as statistically significant.
RESULTS
Of 32 patients, 19 were females and 13 were males. The mean age of the patients was 40.3±13 years with a range of 16-62 years. Fifteen right and 17 left ears were operated. Mean follow-up period was between 6-24 months, with a mean of 12.4±3.7 months.
The cause of perforation was ventilation tube removal in five, trauma in five, otitis media sequel in 14, and graft reperforation or insufficiency despite successful hearing outcomes after microscopic tympanoplasty in eight patients. All perforations were central, and located in the anterior quadrant, and their size ranged between 2-5 mm. There was anterior wall protrusion in 15 patients, and the anterior annulus could not be seen.
A total of seven patients, one child and six patients who could not tolerate local anesthesia had surgery under general anesthesia, and the remaining 25 patients were operated under local anesthesia. The duration of surgery was between 27-46 minutes, and the mean duration for surgery was 36.1±5.82 minutes.
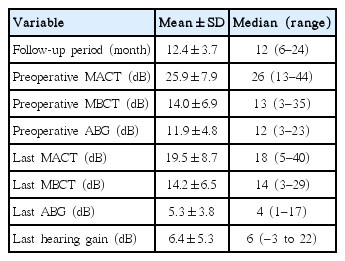
Preoperative mean air conduction threshold of 32 patients was 25.9 dB, mean bone conduction threshold was 14 dB, and mean ABG was 11.9 dB, and these values improved to 19.5 dB, 14 dB, and 5.3 dB, respectively in their last audiograms. The mean hearing gain was 6.4 dB. Closure of ABG to 10 dB or lower was seen in 91% of the patients (Table 1).
Statistical analysis revealed that the difference between preoperative and last postoperative mean air conduction thresholds was significant (P=0.001). The comparison of preoperative and last postoperative ABG also yielded statistically significant results (P=0.001).
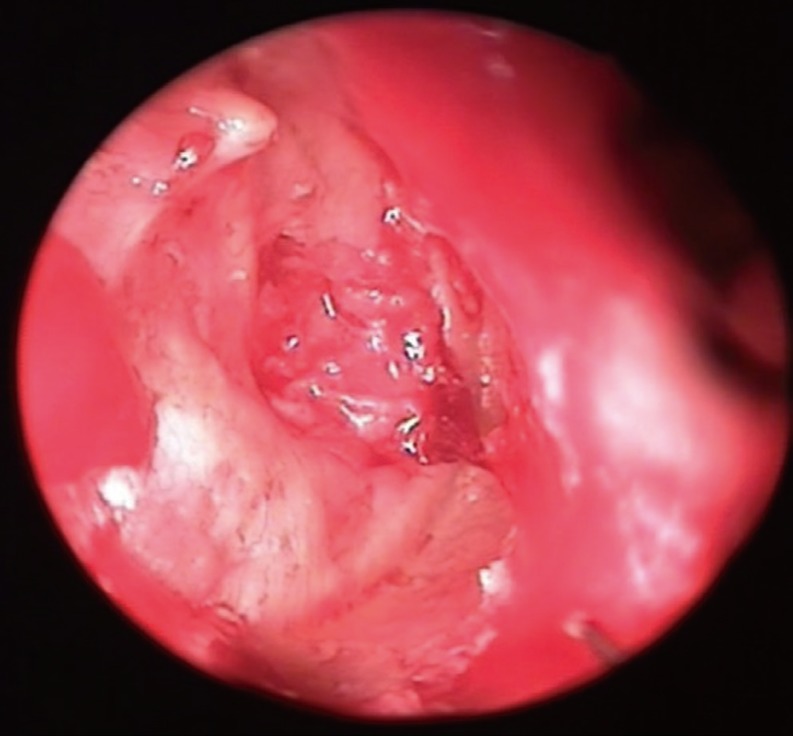
Graft success rate was 87.5%. In one patient with EPTCM, it was seen that the cartilage graft slipped from the superior edge of the perforation inferiorly, and covered 50% of the primary perforation three weeks after surgery. Fat myringoplasty was preformed in postoperative second month in this patient. The perforation was seen to be closed at postoperative 4th month. In one patient, a 1-mm-sized perforation was seen at the posterior edge of the perforation at postoperative 2nd month. This perforation was seen to be closed spontaneously at postoperative 6th month. These two patients were classified as graft failure in the study. In one patient, a pearl cholesteatoma was seen at the posterior edge of the perforation, and it was removed by the endoscopic approach. Fig. 4 demonstrates the pearl cholesteatoma, and the postoperative endoscopic appearances of some patients.
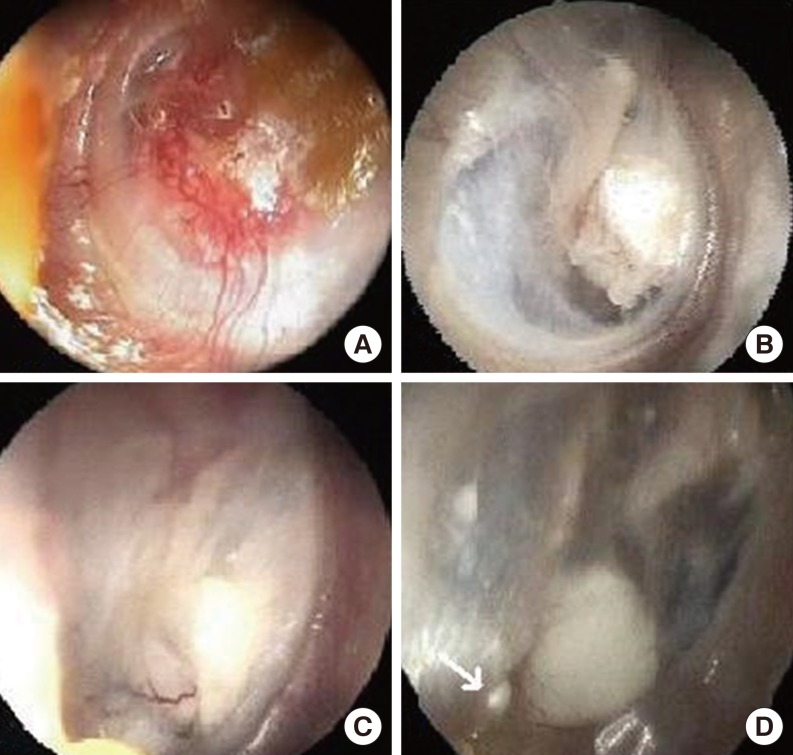
Postoperative endoscopic appearances of the patients. (A) The appearance of vascularization on the cartilage graft at second postoperative month, left ear; (B) The appearance of the cartilage after one year postoperatively, right ear; (C) The appearance of cartilage anterior to malleus manibrium, and the vascular structures feeding it at postoperative 6th month, right ear; (D) The appearance of the pearl cholesteatoma (white arrow) placed at inferior-posterior end of the intact cartilage graft at postoperative 6th month, right ear.
Except one patient with a cholesteatoma pearl, other patients did not have minor or major complications related to surgery.
DISCUSSION
A number of techniques have been described for repair of anterior TM perforations. Traditional approach for these perforations include underlay placement of a graft beneath a tympanomeatal flap prepared with skin incisions. Anterior canalplasty is performed as an additional intervention when anterior canal wall is prominent, and anterior annulus cannot be observed. In the technique described herein, the graft can be placed through the perforation by a transcanal underlay approach without any need for skin incisions or preparation of a tympanomeatal flap. A prominent anterior canal wall, and inability to see the anterior edge of the perforation and annulus make myringoplasty difficult when an operation microscope is used. Use of endoscopes provides advantages to surgeon in these circumstances. Microscope magnifies the surgical field on a straight line, however endoscopes magnify the area at a wider angle. Deep corners can be visualized by endoscopes [25]. Therefore, anterior annulus can be easily seen, and the intervention can be performed without any need for an anterior canalplasty. Since magnification is achieved by getting the endoscope closer to the surgical area, manipulation of the endoscope is easier and faster compared to the microscope. Use of endoscopic push-through technique in anterior TM perforations have some advantages over other techniques such as no need for skin incisions, less bleeding, a short operation time, ease of application, and no need for postoperative care. In our study, we used endoscopes to take these advantages. However in anterior quadrant perforations, if the anterior wall is prominent, the problem is absence of suitable standard instruments designed for endoscopic surgery of this region. In this study, we changed the angles of the picks, and used them particularly to gain access to difficult-to-reach areas. Since the endoscope is held with the one hand, and one-hand surgery seems as a disadvantage of endoscopic surgery, however the procedure can easily be performed with one hand as experience is gained and bleeding is controlled well.
Anterior quadrant perforations are risky perforations. In these patients, cartilage may be preferred to other graft materials (fat, temporal fascia or perichondrium), since it is a strong material both against absorption and negative pressure produced by maneuvers such as sniffing [678]. In transcanal approach, tragal cartilage is preferred instead of temporal muscle fascia since tragal cartilage is more practical to obtain, and to avoid an incision scar. This is why perforations of 8 patients were regarded as risky perforations in this study because they had revision surgeries, and tragal cartilage was preferred as a graft material. In butterfly technique where a cartilage graft is used, preparation of the graft (such as shaping the edges of the graft one-to-one for the perforation) is harder and more time consuming compared to the push-through technique. In this technique, the cartilage graft does not need to be shaped, and it is just cut 2-3 mm larger than the perforation's diameter, and placed underlay through the perforation. The maximum perforation for this technique must be approximately 5 mm×5 mm, and it may reach to annulus anteriorly and inferiorly, 1 mm away from malleus manubrium posteriorly, and 2-3 mm away from anterior malleolar ligament superiorly. Fat is not a suitable graft material for the perforations of this size. In our study, the perforation sizes ranged between 2-5 mm.
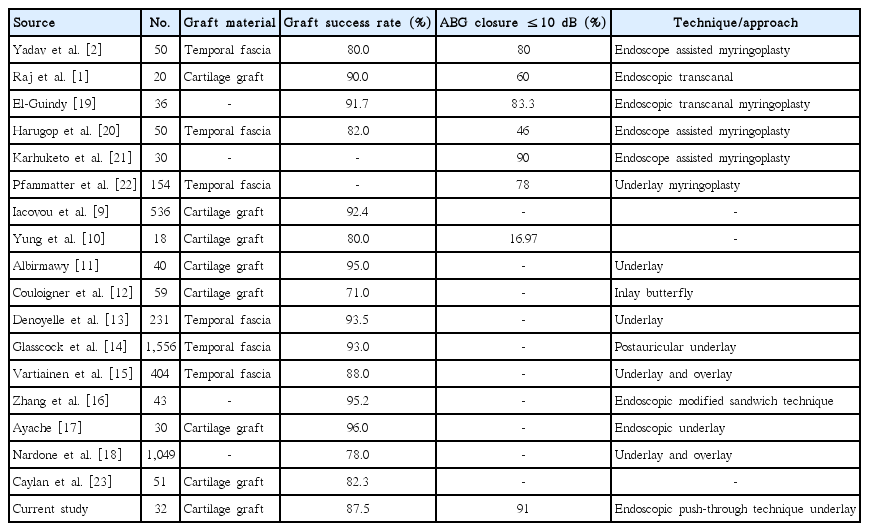
Myringoplasty is a surgical approach which is used for repairing TM perforations when the ossicular chain is intact. Therefore, hearing is not bad in these cases. Myringoplasty is also performed in mixed type hearing loss when both air and bone conduction thresholds are low, and ABG is small. Therefore, the hearing gain in myringoplasty is not as good as predicted in the tympanoplasty. In this study, preoperative mean air conduction threshold was 25.9 dB and preoperative mean ABG was 11.9 dB, and these values improved to 19.5 dB and 5.3 dB, respectively in the last audiograms obtained. The statistical analysis of these parameters yielded significant results. The hearing results obtained by endoscopic push-through technique cartilage myringoplasty in this study, and the myringoplasty results in the literature are compared in Table 2 [1291011121314151617181920212223].
The graft success rate in myringoplasty was reported as 71%-96% in the literature [1291011121314151617181920212223]. In this study, the graft success rate obtained by endoscopic push-through technique cartilage myringoplasty was 87.5%, and it is comparable with the literature.
The advantages of endoscopic push-through technique cartilage myringoplasty are; no need for a flap elevation or skin incision except the incision performed for obtaining the graft, short operation time, no need for a mastoid dressing postoperatively, fast postoperative wound healing, patient comfort and good cosmetic results.
In conclusion, endoscopic push-through technique underlay cartilage myringoplasty has anatomical and functional results comparable with the literature, and it is an effective, minimally invasive, and feasible method.
Notes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.