Anatomical Factors Influencing Pneumatization of the Petrous Apex
Article information
Abstract
Objectives
Aim of the present study was to define the relationship between petrous apex pneumatization and the nearby major anatomical landmarks using temporal bone computed tomography (CT) images.
Methods
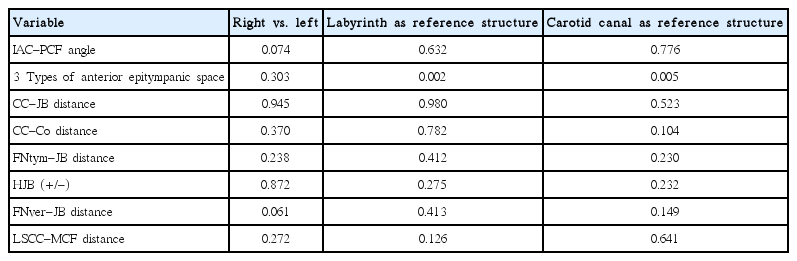
This retrospective, Institutional Review Board-approved study analyzed CT images of 84 patients that showed normal findings bilaterally. Pneumatization of the petrous apex was classified using two methods. Eight parameters were as follows: angle between the posterior cranial fossa and internal auditory canal, Morimitsu classification of anterior epitympanic space, distance between the carotid canal and jugular bulb, distance between the cochlear modiolus and carotid canal, distance between the tympanic segment and jugular bulb, high jugular bulb, distance between the vertical segment and jugular bulb, and distance between the lateral semicircular canals and middle cranial fossa.
Results
There was a significant difference in Morimitsu classification of the anterior epitympanic space between the two classification methods. Poorly pneumatic upper petrous apices were distributed uniformly in three types of Morimitsu classification, but more pneumatic upper petrous apices were found more often in anterior type. Lower petrous apex was well pneumatized regardless of the types of anterior epitympanic space, but the largest amount of pneumatization was found more frequently in the anterior type of anterior epitympanic space.
Conclusion
This study showed that there was no reliable anatomic marker to estimate petrous apex pneumatization and suggests that the pneumatization of the petrous apex may be an independent process from other part of the temporal bone, and may not be influenced by the nearby major anatomical structures in the temporal bone. In this study, the anterior type of anterior epitympanic space was found to be closely related to more well-pneumatized petrous apices, which implies that the anterior saccule of the saccus medius may be the main factor influencing pneumatization of the petrous apex.
INTRODUCTION
Pneumatization refers to the development of air-filled cavities in bone. Within the temporal bone, pneumatization develops primarily through the Eustachian tube to the middle ear and mastoid process. According to the literature, the development of otitis media and the formation of cholesteatomas may be related to the degree of pneumatization of the temporal bone. However, it is not known whether poor pneumatization is the cause or the result of otitis media [12].
Anatomically, the petrous apex is the most medial portion of the temporal bone and composed of dense bone and bone marrow. Generally, its boundaries are the petro-sphenoidal fissure and internal carotid artery anteriorly, the posterior cranial fossa (PCF) posteriorly, the petro-occipital (petroclival) fissure medially, and the inner ear medially. Its pneumatization occurs by the posterosuperior and posteromedial cell tracts when epithelium-lined air cells develop medially from the mastoid air cells. Unfortunately, the petrous apex displays anatomic variations, such as asymmetric pneumatization, that might be mistaken for underlying lesions [34].
Abnormal pneumatization of the petrous bone may be responsible for some clinical symptoms. It has been reported that patients with its abnormal pneumatization are more prone to develop complications. Yamakami et al. [5] also reported that the extent of the mastoid cells was significantly correlated with the pneumatization of the petrous apical cells. They found that petrous air cells can provide the route for cerebrospinal fluid (CSF) rhinorrhea and suggested that CT assessment of the petrous air cells could be useful for preventing CSF rhinorrhea after the skull base surgery.
In the current literature, few studies have used CT imaging to study pneumatization of the petrous apex, so data on pneumatization in this region are limited [346]. Thus, the aim of the present study was to define the relationship between petrous apex pneumatization and major anatomical landmarks using temporal bone CT imaging. This study also assessed which anatomical landmarks in the temporal bone may be factors influencing the pneumatization of the petrous apex.
MATERIALS AND METHODS
For this retrospective analysis, we collected all of the temporal bone CT images that were taken at the university-based hospital from January 2013 to January 2014. Only adult patients 19 years and older were included in the study. The exclusion criteria of this study were as follows: (1) cases where any type of otitis media or cholesteatoma were diagnosed during the physical exam, (2) cases showing sclerotic changes in the temporal bone, including in the petrous apex, (3) cases of temporal bone fractures, and (4) cases that underwent previous tympanomastoidectomy or other mastoid surgeries. Non-contrast-enhanced, high-resolution, multislice CT examinations (GE Medical System, Milwaukee, WI, USA) of the temporal bones were performed using the following parameters of acquisition: collimation width of 1.0 mm, 120 kV (peak), and 340 mA.
Pneumatization of the petrous apex was classified into four groups using two classification methods. One method used the labyrinth as a marker, and the second method used the carotid canal (CC) as a marker [7]. In the group that used the labyrinth as a marker, cases were further subclassified into four groups to evaluate the degree of pneumatization as follows: (1) group 1 had no air cells seen in the vicinity of the inner ear, (2) group 2 had pneumatization of less than half of the petrous apex medial to the labyrinth, (3) group 3 had pneumatization of more than half of the petrous apex medial to the labyrinth, and (4) most of the petrous apex area medial to the labyrinth was composed of air cells in group 4 (Fig. 1A, B). Using the CC as the reference for evaluation of temporal bone pneumatization, cases were categorized into four groups as follows: (1) group 1 had no air cells present at the petrous apex, (2) group 2 had some air cells in the vicinity of the CC, (3) group 3 had air cells detectable at the petrous area lateral to the CC only, and (4) group 4 had detectable air cells at the petrous area medial to the CC (Fig. 1C, D).

Examples of petrous apex pneumatization classification. Group 2 (A) and group 3 (B) classified using the labyrinth as the reference structure. Group 3 (C) and group 4 (D) classified using the carotid canal as the reference structure.
On axial imaging, the following three parameters were measured: (1) IAC-PCF angle (degree), (2) type of anterior epitympanic space, (3) CC-JB distance (mm). On axial imaging, which showed the clearest view of the internal auditory canal (IAC), the IAC-PCF angle was measured between the arbitrary axis of the IAC and an arbitrary line drawn at the center of the IAC porus and at the most medial aspect of the sigmoid sinus (Fig. 2A). The anterior epitympanic space was classified into three types according to Morimitsu classification [89]. On axial imaging, which showed ascending sections of the petrous segment (C2) of the CC just below its bend section, the CC-JB distance was measured to be the shortest distance between the CC and the most medial aspect of the jugular bulb (JB) (Fig. 2B). In cases of low JBs, the CC-JB distance was measured to be the shortest distance between the CC and the posterior cranial fossa (Fig. 2C).
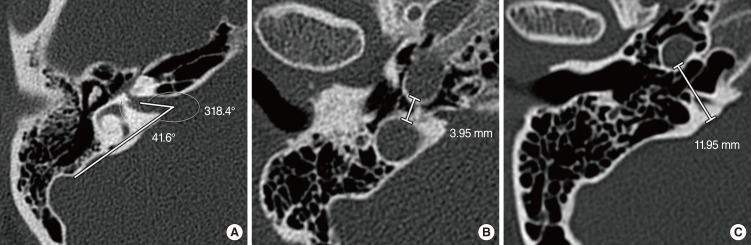
Measurements of IAC-PCF angle (A) and CC-JB distance (B, C). IAC, internal auditory canal; PCF, posterior cranial fossa; CC, carotid canal; JB, jugular bulb.
On coronal imaging, the following five parameters were measured: (1) CC-cochlea (Co) distance (mm), (2) FNtym-JB distance (mm), (3) the presence or absence of a high JB (HJB [+/-]), (4) FNver-JB distance (mm), (5) LSCC-MCF distance (mm). On coronal imaging, which showed the clearest view of the Co, the CC-Co distance was measured to be the shortest distance between the CC and the Co modiolus (Fig. 3A). The FNtym-JB distance was measured to be the shortest distance between the tympanic segment of the facial nerve (FNtym) and the JB (Fig. 3B). On coronal imaging, which showed the clearest image of the stylomastoid foramen, the FNver-JB distance was measured to be the shortest distance between the most medial aspect of the vertical (mastoid) segment of the facial nerve (FNver) and the most lateral aspect of the JB (Fig. 3C). On coronal imaging, which showed the clearest image of the lateral semicircular canal (LSCC), the LSCC-MCF distance was measured to be the shortest distance between the uppermost aspect of the LSCC and the middle cranial fossa (Fig. 3D).

Measurements of CC-Co distance (A), FNtym-JB distance (B), FNver-JB distance (C), and LSCC-MCF distance (D). CC, carotid canal; Co, cochlea; FNtym, tympanic segment of the facial nerve; JB, jugular bulb; FNver, vertical (mastoid) segment of the facial nerve; LSCC, lateral semicircular canal; MCF, middle cranial fossa.
All statistical analyses were performed using IBM SPSS ver. 19.0 (IBM Co., Armonk, NY, USA). Data were described using the mean±standard deviation. P-values less than 0.05 were considered to be significant (two-tailed significance).
The Institutional Review Board of Uijeongbu St. Mary's Hospital (IRB No. UC14RISI0040) approved this study. Informed consent for the CT images was obtained for all participants.
RESULTS
This study analyzed the CT images of 84 patients (37 males and 47 females) with a mean age of 43.9±10.2 years (range, 20 to 62 years). The mean values of the six measured parameters are as follows: IAC-PCF angle (47.3±7.0 degrees), CC-JB distance (6.3±4.7 mm), CC-Co distance (8.2±2.9 mm), FNtym-JB distance (13.9±3.0 mm), FNver-JB distance (7.3±2.8 mm), LSCC-MCF distance (8.7±1.2 mm).
There were no significant differences in any of the eight measured parameters between the right and left sides (Wilcoxon signed rank test) (Table 1). Additionally, there were no significant differences in IAC-PCF angle, CC-JB distance, CC-Co distance, FNtym-JB distance, FNver-JB distance, or LSCC-MCF distance between the four subgroups in either of the two classification methods (one-way analysis of variance) (Table 1). There were no significant differences in HJB (+/-) between the subgroups in either classification method (chi-square test) (Table 1). However, there were significant differences in the types of anterior epitympanic space between the subgroups in either classification method (chi-square test) (Table 1). Generally, the upper petrous apex was more pneumatic than the lower part. The proportion of poorly pneumatic upper petrous apex was even between the three types of anterior epitympanic spaces. The upper petrous apex was much less pneumatized in the plate and pyramid types than in the anterior type of anterior epitympanic space. The lower petrous apex was well pneumatized regardless of the type of anterior epitympanic space, but greater levels of pneumatization were found more often in the anterior type of anterior epitympanic space (Fig. 4). There was a significant correlation between both classification methods of anterior epitympanic space (chi-square test, P<0.001; gamma, 0.631).

Bar graphs showing significant differences in the types of anterior epitympanic spaces between both classification groups. (A) Poorly-pneumatic upper petrous apices of group 1 were distributed uniformly in the three types of anterior epitympanic space, but better-pneumatized upper petrous apices were found more often in the anterior type. (B) Lower petrous apices were well pneumatized regardless of the type of anterior epitympanic space, but the most pneumatization of group 4 was found more frequently in the anterior type of epitympanic space. AES, anterior epitympanic space.
DISCUSSION
The objective of this study was to evaluate the factors that can influence the pneumatization of the petrous apex, which has been a subject of controversy and has not been widely studied. The petrous bone can be divided into two main compartments, anterior and posterior, in relation to the IAC and the otic capsule. The anterior compartment, known as the petrous apex, is the larger compartment of the two. Pneumatization of the petrous apex can vary, and about one third of adults do not have pneumatization. The shape of the petrous apex is pyramidal, with an anterior, posterior, and inferior surface. The apex is located between the clivus on its anteromedial side and the inner ear on its posterolateral side. The petrous apex is a complex structure that both includes and abuts several major anatomical structures. For example, the apex's anterior surface forms the floor of the MCF, and the internal carotid artery passes through the CC, which is within the petrous apex, into the cavernous sinus. Specifically, the opening of the CC and JB are located on the apex's inferior surface. In addition, the Eustachian tube, together with the tensor tympani muscle, is located just lateral to the CC. The apex's posterior surface faces the PCF, and the superior and inferior petrosal sinuses are located along superior and inferior edges of posterior surface.
In this study, the labyrinth as well as CC was used as a reference landmark to classify the grade of temporal bone pneumatization. The reason why these two structures were chosen was that these two are major anatomical structures in the temporal bone, which are not influenced by the pneumatization process within the temporal bone. This is based on the developmental difference of these structures. In addition, the classification referenced on these two structures can evaluate the intricately shaped petrous apex effectively because of their different locations within the temporal bone.
Pneumatization of the petrous apex is different than pneumatization of the middle ear and mastoid air cells. Developmentally, the mastoid air cells represent an extension of the air tracts in the middle ear cleft through the Eustachian tube from the first pharyngeal pouch. Pneumatization in the mastoid extends from the middle ear cleft through the aditus ad antrum to the central air cell tract from which further extension in several directions may occur. Two main tracts in the mastoid form the anterolateral part (the squamous portion) and posteromedial part (the petrous portion) of the temporal bone. The incomplete partition between these two tracts is called the petrosquamous septum, also known as the Koerner septum. The petrous apex develops from the posterosuperior and posteromedial cell tracts. The posterosuperior cell tract extends medially at the level of the superior semicircular canal, and the posteromedial cell tract extends at the level of the posterior semicircular canal. Some authors stated that the subarcuate cell tract, the perilabyrinthine cell tract, and the peritubal cell tract can also participate in the pneumatization of the petrous apex to varying degrees [1011].
The aim of this study was to explore which factors influence petrous apex pneumatization. Because the pneumatization of the temporal bone has been known to be influenced by hereditary and environmental factors, this study included only adult cases in which the mastoid bone was well pneumatized to reduce the bias of environmental influence. Our initial hypothesis was that variation of major anatomical structures within the petrous apex may influence the development of the posterosuperior, posteromedial, subarcuate, perilabyrinthine and peritubal cell tracts, which might result in various levels of pneumatization of the petrous apex. However, this study failed to reveal any anatomical relationship between the IAC, intracranial cavity (MCF and PCF), vascular structures (CC and JB), otic capsule (CC and LSCC), and facial nerve and the pneumatization of the petrous apex. These results suggest that the petrous apex might be developmentally independent during its pneumatization.
This study demonstrated significant differences in the types of anterior epitympanic space between the subgroups in either classification method. This study showed that well pneumatized petrous apices were generally found in the anterior type of anterior epitympanic space. In the upper petrous apex part, well pneumatic cases of groups 2-4 were found mainly in anterior type of anterior epitympanic space. In the lower portion of the petrous apex, well pneumatic cases of group 4 were found mainly in the anterior type of the anterior epitympanic space. This suggests that the anterior type of anterior epitympanic space is closely related the well pneumatic petrous apex. The anterior epitympanic space is determined during the embryologic development of the saccus anticus and the anterior saccule of the saccus medius. In particular, the anterior saccule of the saccus medius is critical for the development of the anterior type of anterior epitympanic space [121314]. Therefore, we carefully infer that the anterior saccule of the saccus medius may be the main factor in the pneumatization of the petrous apex.
Anterior epitympanic space has many different names. Proctor called the airspace anterior to the malleal head as the supratubal recess. Wigand and Trillsch named it as the sinus epitympani. It was also known as anterior epitympanic compartment, anterior attic recess, recessus protympanicum, anterior epitympanic recess, and geniculate sinus in many literatures [12]. It is bordered superiorly by anterior part of the tegmen tympani, inferiorly by cochleariform process and tensor tympani fold, anteriorly by the zygomatic root, posteriorly by the cog, laterally by the scutum, and medially by anterior portion of the tympanic facial nerve and geniculate ganglion. Its development is different from that of the middle ear and mastoid air cells. Anterior epitympanic space is developed by upward expansion of the aeration from the bony Eustachian tube, which begins at a late fetal stage and continues throughout childhood. However, the aeration of the middle ear cavity and the mastoid antrum is complete by birth [1214]. The other evidence suggesting that it develops independent of the mastoid air cell system is that the anterior epitympanic space had formed in 68% of temporal bones without pneumatization of the temporal bone [1516].
In conclusion, this study suggests that pneumatization of the petrous apex may be an independent process from those of the middle ear and mastoid air cells, and may not be influenced by major anatomical structures nearby. The anterior type of anterior epitympanic space is associated with a well pneumatized petrous apex, and the anterior saccule of the saccus medius might be a major influencing factor in the pneumatization of the petrous apex.
Notes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.