Effectiveness of Recombinant Human Growth Hormone for Pharyngocutaneous Fistula Closure
Article information
Abstract
Objectives
In laryngeal cancer, which comprises 25% of head and neck cancer, chemotherapy has come into prominence with the increase in organ-protective treatments. With such treatment, salvage surgery has increased following recurrence; the incidence of pharyngocutaneous fistula has also increased in both respiratory and digestive system surgery. We investigated the effects of recombinant human growth hormone on pharyngocutaneous fistula closure in Sprague-Dawley rats, based on an increase in amino acid uptake and protein synthesis for wound healing, an increase in mitogenesis, and enhancement of collagen formation by recombinant human growth hormone.
Methods
This study was experimental animal study. Forty Sprague-Dawley rats were separated into two groups, and pharyngoesophagotomy was performed. The pharyngoesophagotomy was sutured with vicryl in both groups. Rats in group 1 (control group) received no treatment, while those in group 2 were administered a subcutaneous injection of recombinant human growth hormone daily. On day 14, the pharynx, larynx, and upper oesophagus were excised and examined microscopically.
Results
Pharyngocutaneous fistula exhibited better closure macroscopically in the recombinant human growth hormone group. There was a significant difference in collagen formation and epithelisation in the recombinant human growth hormone group compared to the control group.
Conclusion
This study is believed to be the first in which the effect of recombinant human growth hormone on pharyngocutaneous fistula closure was evaluated, and the findings suggest the potential of use of growth hormone for treatment of pharyngocutaneous fistula.
INTRODUCTION
Pharyngocutaneous fistula (PCF) is a serious and common complication that may occur following respiratory and digestive system surgery. The incidence of PCF following total laryngectomy is 3%-15%; it is the most common complication [12]. PCF is seen mostly at 4-10 days postoperatively [3]. It has been reported that the most important risk factors for PCF development are nutritional deficiency, neck dissection in addition to laryngectomy, preoperative tracheotomy, infection, diabetes, anaemia, tumour dimensions, preoperative chemotherapy, postoperative hypovolaemia, and positive surgical margins [4]. The most important preoperative risk factor is neck irradiation. In patients who have undergone preoperative radiotherapy, the incidence of PCF is 2.6 folds higher than in those who have not undergone radiotherapy [5]. When PCF occurs, the first measures to take are: discontinuation of oral intake, nasogastric and salivary bypass tube placement, fistula tract tampon with antiseptic gases, appropriate antibiotic treatment, and attempted increase in granulation tissue [67]. Despite these precautions, PCF does not close in 30% of patients and surgical intervention is required [8].
Secretion of growth hormone (GH) from the pituitary gland is maximal during puberty and decreases by 25% in old age [910]. GH is an anabolic hormone that increases protein synthesis (tissue growth). It decreases protein and amino acid catabolism, whereas it enhances lipid usage (ketogenic effect). It decreases energy supply from glycogen and amino acids [911]. Some studies have shown that GH levels increase following trauma, haemorrhage, surgery, and analgesia; GH prevents protein loss and regulates nitrogen balance [1213]. It has been proven that recombinant human GH (rHGH) enhances wound recovery and immune function in such situations as major surgery, trauma, sepsis or thermal injury and decreases hypermetabolic response. GH demonstrates a limiting effect on acute phase reactant formation in cases of trauma, surgery and stress, while protein synthesis increases [1415]. GH enhances wound healing while increasing hydroxyproline interaction and tissue tension, especially in dermal and enteric wounds and increase osteoblast proliferation [161718].
Based on its effects on wound healing, we investigated the effectiveness of infused rHGH on fistula closure and histopathological changes in healing tissue following PCF formation in Sprague-Dawley rats after pharyngoesophagotomy.
MATERIALS AND METHODS
Animal preparation and experimental procedures
Approval for the research protocol was obtained from the Animal Ethics Committee of the Marmara University Hospital, Istanbul, Turkey, in April 2012. The study was performed at Marmara University Animal Laboratory using 40 Sprague-Dawley rats that weighed 250-350 g. Animals were sedated with 40 mg/kg ketamine hydrochloride (Ketalar, Eczacibasi, Turkey) and 10 mg/kg xylazine (Rompun, Bayer, Germany) and shaved in the neck area. Rats were laid in the supine position; head extended over the surgical table and a 3-cm vertical incision was made over the neck midline. Skin and subcutaneous tissue was passed. The oesophagus was located by opening through the sternohyoid muscle using rough dissection, followed by lateralisation of the sternohyoid muscle. Pharyngoesophagotomy was performed using 1.5-cm longitudinal incisions on the lateral border of upper oesophagus and pharynx, and between the oral palate and upper oesophagus. The 1.5-cm pharyngoesophagotomy incision was closed loosely with three 8.0 vicryl sutures (Fig. 1) to be able to create a PCF. Lastly, the skin was closed tightly using a 4.0 silk suture (Fig. 1). Rats were then placed in individual cages.
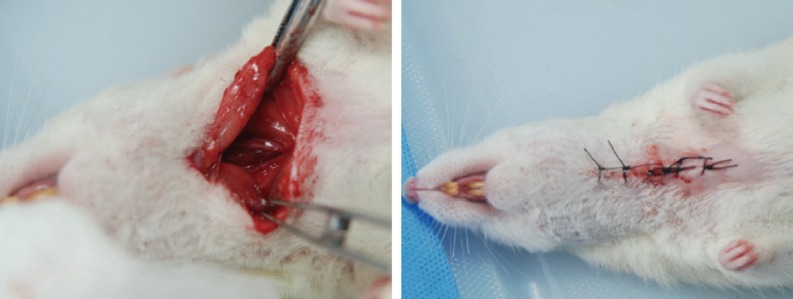
Following a 3-cm vertical incision in the neck midline, subcutaneous muscles were eradicated. Following sternohyoid muscle lateralisation, the oesophagus was located under the trachea. A 1.5-cm incision was then made between the floor of the mouth and the upper oesophagus and sutured with 8.0 vicryl. The skin was closed with 4.0 silk.
Rats were separated into two groups randomly, each comprising 15 male and 5 female rats. All rats were fed with dry prey and water. The control group was injected subcutaneously with 5 mL 5% dextrose (Polyflex 5% dextrose; Polifarma Drug Ind., Çorlu, Turkey) twice a day in the morning and evening for 14 days. Twenty rats in group 2 received 2 mg/kg/day rHGH (Saizen click easy, Merc Serono S.p.A., Italy) and 5 mL 5% dextrose subcutaneously, starting from the first postoperative day for 14 days. Although 5% dextrose was given every day and they were fed with dry prey and water, the rats lost weight fastly day after day. We did not make anything for dressing or saliva control which are important for preventing PCF formation in human body. Changes in the neck area and the presence of leakage or openings were recorded daily. Due to closed very loosely the inner layer and also because of malnutrion, on day 3 or 4, leakage in the neck and wound separation were observed in both groups. Two rats in the control group and three in the rHGH group died before 1 week and were excluded from the study.
Pathological evaluation
Rats were sacrificed after 14 days, and the larynx was extracted without damaging the pharynx, upper oesophagus or skin integrity. Specimens were fixed in formaldehyde for 24 hours and sectioned. Fistula formation between the pharynx and oesophagus and the skin was evaluated macroscopically and scored. Cutaneous, subcutaneous and oesophageal extension of the fistula was evaluated macroscopically and a midline section was taken, blocked and processed for 13 hours in an automatic tissue processor (Shandon Ex-celsior ES, Thermo Scientific, Runcorn, England). Following processing, 2-µm sections were taken from paraffinised tissue and stained with haematoxylin and eosin (H&E). Stained sections were observed with a light microscope (Olympus Bx-50, Olympus Optical, Tokyo, Japon) by a pathologist blinded to the evaluation. Macroscopic PCF closure, fibroblast concentration, collagen formation, and surface epithelisation were evaluated and scored.
Fibroblast concentration was evaluated as follows: fibroblast cell count <5, minimal; 5-15, moderate; 15-50, high; >50, very high. Squamous epithelial cells were identified and epithelised and ulcerated areas were evident. For descriptive statistics, rates and frequencies were used. For variance analysis, IBM SPSS ver. 20.0 (IBM Co., Armonk, NY, USA) was used with a chi-square test, Fisher exact test, as appropriate.
RESULTS
Macroscopic PCF closure
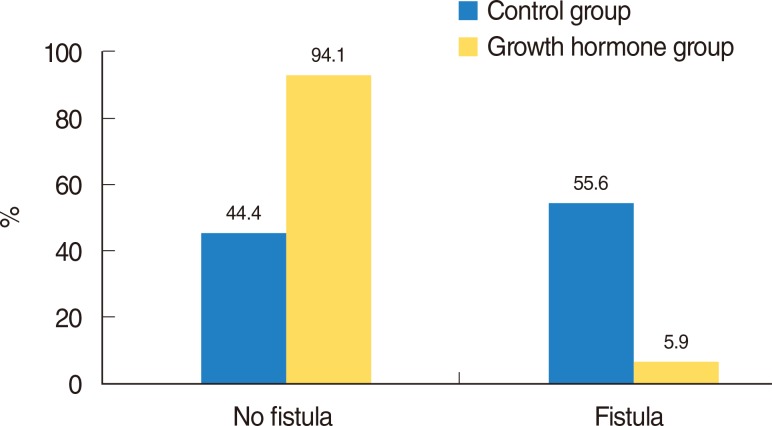
Following sacrifice, macroscopic fistula evaluation in the neck area was carried out before specimens were placed in paraffin blocks. In the rHGH group, one of 17 rats demonstrated an opening in the neck, compared with 10 of 18 rats in the control group. Ulceration and accumulation of leftover food were observed in the neck (Fig. 2). The chi-square test showed that PCF closure in the rHGH group was significantly better than in the control group (P=0.002) (Fig. 3).
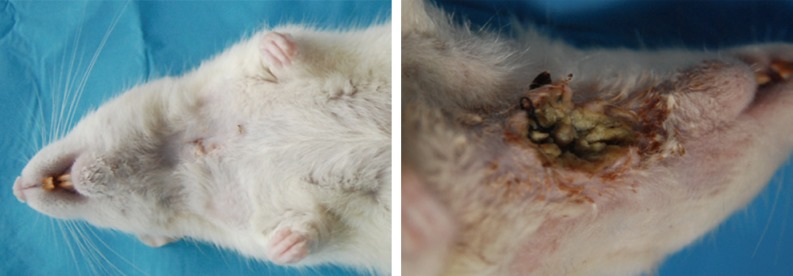
Macroscopic image of a closed fistula in the neck and macroscopic image of abscess formation in the neck.
Fibroblast concentration
Fibroblast concentrations in H&E-stained sections were investigated. Sections were divided into two groups: one had a minimal or moderate number of fibroblasts, and the other had a high or very high number of fibroblasts (Fig. 4). In the control group, 15 of 18 rats and in the rHGH group, 13 of 17 rats showed intense fibroblast concentration in the fistula area. Fisher exact test was performed. A high fibroblast concentration was found in the control and rHGH groups, but there was no significant difference between the fibroblast counts (P=0.691) (Fig. 5).
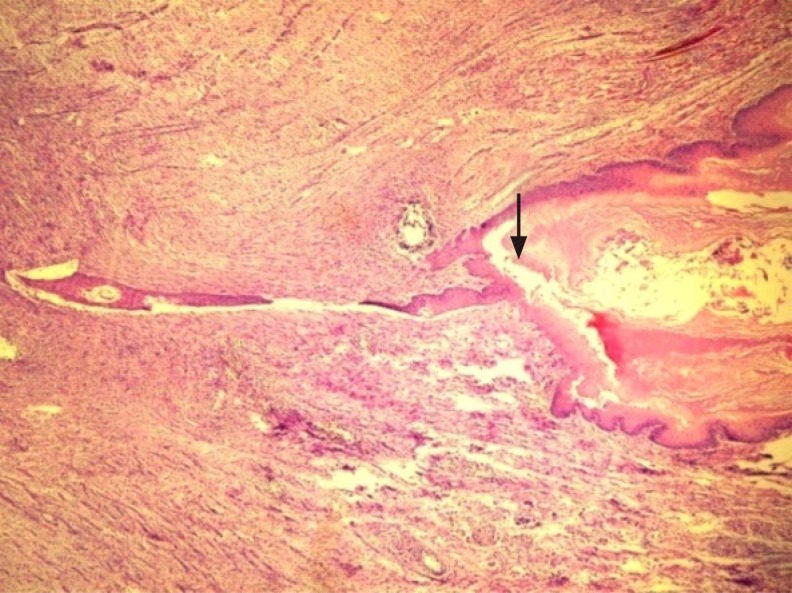
Epithelised fistula tract; decreased inflammatory cells and dense fibroblasts are seen around the fistula tract (arrow) in the control group (H&E, ×100).
Collagen formation
In the control group, 13 of 18 rats showed little collagen formation in the fistula area. In the rHGH group, 11 of 17 rats showed intense collagen formation (Fig. 6). Collagen formation in the rHGH group was significantly higher than that in the control group (P=0.011, chi-square test, Fischer exact test) (Fig. 7).
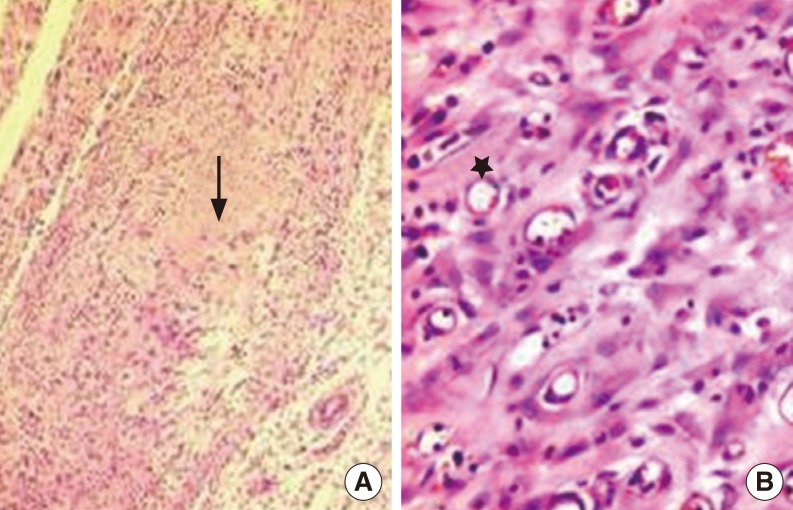
(A) The fibroblasts significantly decreased and collagen tissue accumulation was seen (arrow) in the recombinant human growth hormone group. (B) The granulation tissue rich in small capillary vessels (star) show delayed recovery in the control group (H&E, ×100).
Surface epithelium
Surface epithelium was evaluated histopathologically for ulceration and regeneration and thickening. In the control group, 10 of 18 rats showed ulcer formation in the fistula area. In the rHGH group, only 1 of 17 rats showed ulcer formation. Regeneration and thickening of the surface epithelium was significantly greater in the rHGH group than the control group (chi-square test; P=0.002) (Figs. 8, 9).
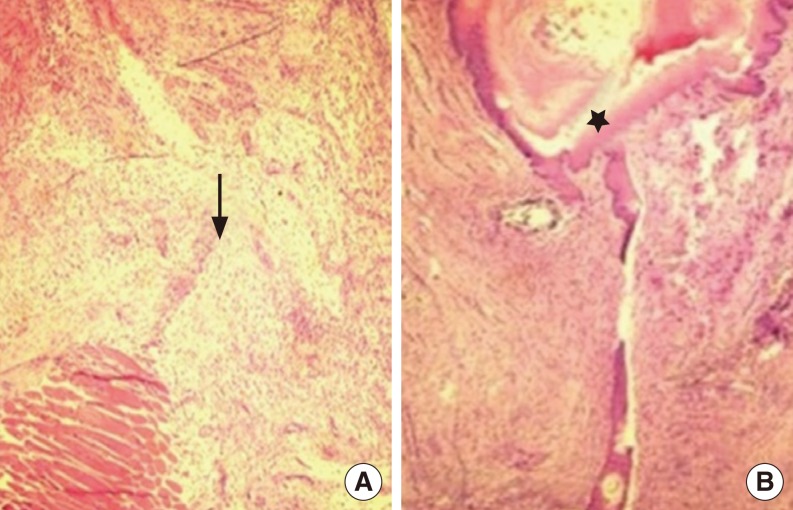
(A) In the recombinant human growth hormone group with no fistula formation, formation of scars rich in collagen (arrow) was seen. (B) A fistula tract was evident with rich fibroblast formation and low inflammatory cell count around the fistula (star) in the control group (H&E, ×100).
DISCUSSION
PCF rates after salvage surgery for cancer relapse have been increased by the use of primary chemoradiotherapy before organ protective surgery in laryngeal and hypopharyngeal squamous cell cancer. To prevent PCF formation following total laryngectomy, many authors have recommended that oral nutrition start between 7 and 10 days. However, recent studies have shown that there is no difference between oral nutrition started on day 2 or 3 and day 7 in terms of PCF formation [19].
In the literature, there are reports of the risk factors and precautions for PCF formation, as well as on fistula closure with a free tissue flap or pectoralis major, deltopectoral or musculocutaneous flaps [2021]. In this study, we investigated the effect of rHGH on fistula closure. We found that, macroscopically and histopathologically, collagen formation and regeneration were more marked in the rHGH group compared with the control group. rHGH had a positive effect on PCF closure. Wiseman et al. [8] closed the mucosa in a patient with FCF using fibrin glue and reported that the PCF was closed at follow-up.
Although rHGH is used commonly in GH-deficient children, recently, it has been used for fracture and wound healing and burn recovery, due to its anabolic effects. GH has also been used for treatment of osteoporosis, bone and cartilage regeneration, burns treatment, and nerve regeneration [6152223]. Although many studies demonstrate the efficacy of GH for tissue epithelisation and wound healing, our study is believed to be the first to use GH for PCF closure. We obtained promising results, with significantly higher collagen formation in the rHGH group.
Similar to our study, Gu et al. [24] injected subcutaneous rHGH into eight patients with enterocutaneous fistula. After 7 days, immunohistochemical evaluation of endoscopic biopsies showed that rHGH enhanced intestinal mucosal epithelial proliferation, protein synthesis and collagen formation. In similar studies, it has been shown that GH is effective in humans and animals with short bowel syndrome. In the present study, collagen formation and regeneration in PCF recovery were significantly better in the rHGH group than the control group. According to macroscopic evaluation, PCF closure was significantly better in the rHGH group than the control group. We demonstrated that rHGH increased regeneration and epithelisation in PCF closure and enhanced wound healing [2223].
In wound recovery, increased fibroblast and fibronectin concentrations are expected, together with collagen formation [922]. In studies of the effects of GH on wound recovery, significant increases in fibroblast concentration were reported in conjunction with collagen formation [22]. In our study, although collagen formation in the rHGH group was significantly greater compared to the control group, there was no difference in fibroblast concentration between the two groups. Nevertheless, the presence of more collagen in the rHGH group and better fistula closure suggests that rHGH inhibits the salivary effect on fibroblasts, because saliva negatively affects wound healing and rHGH increases collagen formation and cell proliferation. Thus the effects of rHGH on PCF, saliva and fibroblasts should be investigated.
Side effects of rhGH replacement therapy in humans include arthralgia, edema, benign intracranial hypertension, rash and pain at injection site, transient fever, insulin resistance, and slipped capital femoral epiphysis [25]. Although it is known that GH enhances mitogenesis and cell proliferation in normal tissues, its effect on neoplastic tissue is not clear yet. Renehan and Brennan [26] state that GH replacement therapy is not associated with tumour recurrence but may increase the risk of de novo cancer. More study is needed to show the definite effect of GH on tumoral tissues.
There are few studies in the literature on formation of PCF in animals. This study is based on two experimental animal studies made by Liu et al. [2728]. In these studies, they observed the normal healing process of pharyngotomy/esophagostomy wounds on 16 rats and demonstrated that the inflammatory response reached its highest level 3 days after pharyngotomy/esophagostomy and diminished after 7 days, based on histopathologic examination. They concluded that 7 days was enough for the observation of histopathological signs of inflammation. Following this data and considering that 7 days of observation might be too short to accurately evaluate the histological improvement in response to rHGH, we conducted some preliminary experiments to find out the maximum period to keep a rat alive after pharyngoesophagotomy. First we performed pharyngoesophagotomy on a rat without making a subcutaneous dextrose injection and we observed that the rat died on 3rd day. Then we performed pharyngoesophagotomy and supplied the rat with subcutaneous dextrose injections twice daily, in which case the rat died after 14 days of the initial operation. Thus we decided to sacrifice the rats 14 days after pharngoesophagotomy in this study. The dressing and saliva control and closing the pharyngoesophagatomy are important factors for preventing PCF on human body. But in this study, because of that the aim of us was to make a PCF, we especially did not use any dressing or saliva control and closed the pharyngoesophagotomy incision too loosely to be able to make a fistula.
This is believed to be the third study using an animal model of PCF. Liu et al. [2728] investigated recovery of Sprague-Dawley rats that had been treated with pharyngoesophagotomy with closure via vicryl, prolene, and fibrin glue. We developed a model of PCF in Sprague-Dawley rats and kept them alive with subcutaneous dextrose injections for 2 weeks. In our study, feeding the rats that underwent pharyngoesophagotomy with liquids instead of solid foods may have been more appropriate. Our present model may act as a guide for future animal studies of PCF.
In conclusion, we showed that rHGH was effective for fistula closure by increasing collagen formation and epithelisation in a rat model of PCF. Wound healing in patients with PCF is more difficult and takes longer compared to other areas such as skin or bone, due to the presence of saliva in the fistula area and migration of oral microorganisms to the wound area. Recent studies of wound recovery and nerve regeneration have shown promising results in terms of the effectiveness of GH and other growth factors in PCF. Nevertheless, it should be remembered that GH may increase mitogenesis and cell growth in residual tumour tissue as well as normal tissue. This is believed to be the first animal study to use rHGH for treatment of PCF. In future, rHGH for PCF treatment may be used in more animal and human studies.
Notes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.