Characteristics of Expression of Matrix Metalloproteinases (MMP-2 and MMP-9) in Glottic Squamous Cell Carcinoma and Benign Vocal Fold Lesions
Article information
Abstract
Objectives
The aim of this study was to investigate expression profile of matrix metalloproteinases (MMP-2 and MMP-9) in glottic squamous cell carcinoma (SCC) and benign vocal fold lesions (BVFLs) and to correlate it with clinical and pathological features.
Methods
The immunohistochemical expression of MMP-2 and MMP-9 was investigated in specimens taken from 217 patients group, including vocal fold polyps (n=39), recurrent respiratory papillomatosis (n=30), laryngeal keratosis (n=36), glottic SCC (n=112), and the normal tissue of vocal fold (n=12, control group). The expression of MMP-2 and MMP-9, both in epithelium and stroma cells, was graded on a semiquantitative scale, ranging from 0 (no expression) to 18 points (high expression).
Results
Expressions of both, MMP-2 and MMP-9 were significantly higher in the glottic SCC group comparing with BVFL group. Significant higher expression of parenchymal MMP-2 (P<0.001) and stromal MMP-9 (P=0.01) was revealed in the group of moderate/poorly differentiated glottic SCC comparing with well differentiated glottic SCC group. Expression of stroma MMP-2 was found to be correlated with nodal metastasis (P=0.030). Expressions of both, MMP-2 and MMP-9 were not correlated with clinical stage, tumor T value, smoking, alcohol use, age in the glottic SSC patients group. The MMP-2 stroma value of 11.2 points was determined as the optimum point (limiting value) for separating BVFL and glottic SCC patient groups.
Conclusion
Our results suggest that expressions of both MMP-2 and MMP-9 are up-regulated already in the development of BVFL, the next determinant step is concerned with occurrence of malignization. Limiting value of stroma MMP-2 demonstrates prognostic importance of MMP-2 in glottic SCC carcinogenesis.
INTRODUCTION
Laryngeal squamous cell carcinoma (SCC) constitutes approximately 2%-3% of all malignant tumors. According to the morbidity rate, this tumor ranks second among the malignant tumors of the respiratory system after the lung cancer [1]. Laryngeal carcinoma is one of the most common of head and neck malignant tumors, and glottic SCC constitutes about 66% of all cases of laryngeal cancer [2].
Even though the five-year survival rates of patients with glottic carcinoma are higher than those with malignant tumors of other anatomic laryngeal areas, and distant metastases are quite rarely diagnosed, the risk of local cancer recurrence remains a serious problem [3].
Remodeling of the extracellular matrix (ECM) occurs during the process of malignant tumor formation. During the initial phase of carcinoma cell invasion and metastasis formation, malignant cells begin to spread and infiltrate into the surrounding normal tissues and to degrade the elements of ECM and basement membrane, including type IV collagen, laminin and fibronectin [4]. Matrix metalloproteinases (MMPs), a family of proteolytic zinc- and calcium-containing enzymes, play one of the most important roles in the aforementioned process being implicated in physiological and pathological remodeling of the tissues. Among more than 23 members of the MMPs family, gelatinase-A (MMP-2) and gelatinase-B (MMP-9) seem to be proven having the capacity to degrade the most important elements of EMC and basement membrane, thus contributing to the invasion and metastases of malignant tumors [4,5,6,7].
Increased expression of these MMPs is found in the process of the development of many malignant tumors, including laryngeal carcinoma [8,9,10,11,12,13]. Therefore, most of previous studies have focused on the prognostic value of MMPs expression in laryngeal SCC growth and spreading to the lymph nodes [4,10,12,13,14]. Moreover, previous studies have concentrated on investigation of MMPs expression in entire laryngeal carcinoma, or emphasized peculiarities only of supraglottic carcinoma. Exceptionally, isolated glottic SCC has received less attention.
Until now a few studies have been carried out to investigate the expression of MMPs in benign and premalignant laryngeal lesions providing comparative analysis [9,11,15]. However, this could be important to increase our understanding of role of MMPs in malignant tumor formation and might improve understanding of the molecular events leading to tumor genesis and development.
The aim of this study was to investigate by immunohistochemistry expression of MMP-2 and MMP-9 in glottic SCC and benign vocal fold lesions (BVFLs) and to correlate expression profiles with clinical and pathological features.
MATERIALS AND METHODS
Patients
The total study group consisted of 217 patients treated at the Department of Otorhinolaryngology of Lithuanian University of Health Sciences (LUHS), Kaunas, Lithuania and the Oncological Hospital of the LUHS.
The BVFLs group consisted of 105 patients and included: vocal fold polyps (VFP) subgroup (n=39; median age, 43 years [range, 21 to 69 years]); recurrent respiratory papillomatosis (RRP) subgroup (n=30; median age, 38 years [range, 21 to 83 years]); laryngeal keratosis (LK) subgroup (n=36; median age, 54 years [range, 34 to 78 years]). The clinical diagnosis of nonmalignant laryngeal lesions was based on typical clinical signs revealed during video laryngostroboscopy and direct microlaryngoscopy. All the patients underwent endolaryngeal microsurgical interventions; therefore, the final diagnosis was proven by the results of histological examination of the removed mass lesions of vocal folds.
The newly diagnosed glottic SCCs patients without any previous treatment were included in this study, because due to anatomical peculiarities supraglottic and subglottic cancer could represent different biological characteristics and clinical behavior. Thus, the glottic SCC group comprised 112 patients (105 males and 7 females). The age of the patients ranged from 36 to 79 years (median, 62 years). Clinical diagnosis of glottic SCC was based on data of video laryngostroboscopy and direct microlaryngoscopy, and the final diagnosis was confirmed after pathohistological investigation. The glottic SCC patients underwent standard additional diagnostic tests, including: chest X-ray examination, neck and abdomen ultrasonography, computed tomography of the larynx and neck. The following clinical features of glottic SCC were evaluated: patient's age, gender, smoking and alcohol habits, SCC staging, tumor differentiation grade, neck lymph node status (Table 1).
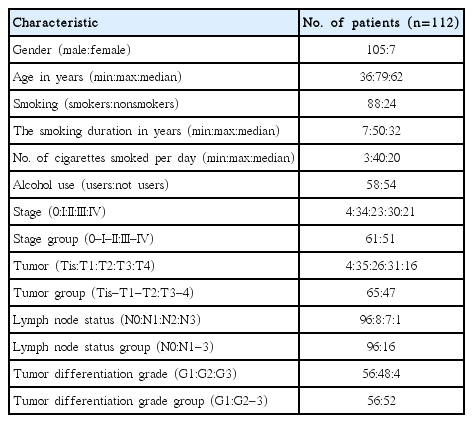
The main clinical and pathological characteristics of glottic squamous cell carcinoma patients group
Control group representing normal vocal fold tissue consisted of 11 females and 1 male, whose age ranged from 23 to 55 years (median, 39 years). Formalin-fixed and paraffin-embedded samples of normal vocal fold tissue were retrieved from the archives of the Department of Pathology of LUHS and served as a control group. These samples represented the cases, where patients underwent endolaryngeal microsurgery for clinically diagnosed "soft" vocal fold nodules and/or Reinke's edema. However, histological examination of the removed specimens revealed "normal tissues of vocal fold".
Protocol of the study was approved by the Ethics Committee for Biomedical Research of Kaunas region.
Immunohistochemical detection of MMP
Formalin-fixed and paraffin-embedded tissue samples of the patients groups and control group were retrieved from the archives of the Department of Pathology of LUHS. Immunohistochemical detection of MMPs was carried out at the Laboratory of Molecular Cardiology in the Institute of Cardiology, LUHS. Three micrometer thick sections were deparaffinised and rehydrated by slide stainer Shandon Varistain Gemini (Thermo Fisher Scientific Inc., Waltham, MA, USA). Then sections were washed with distilled water and heated in Tris/ethylenediaminetetraacetic acid buffer (pH 9.0) for 8 minutes at 110℃ in Microwave Histoprocessor RHS-1 (Microwave Laboratory Systems. Milestone Inc., Shelton, CT, USA). Shandon Coverplate system was used for immunohistochemical labeling. After blocking the activity of endogenous peroxidases, all slides were incubated in the primary antibody buffer for 1 hour at a dilution of 1:100 for MMP-2 (clone 17B11, Novocastra Reagents, Leica Biosystems, Wetzlar, Germany) and MMP-9 (clone 15W2, Novocastra Reagents, Leica Biosystems) in antibody diluent (DakoCytomation, Carpinteria, CA, USA), followed by sequential 30-minute incubations with Advance HRP Link and Advance HRP Enzyme (DakoCytomation). The binding of antibodies was detected by Liquid DAB+Substrate Chromogen System (DakoCytomation). Finally, the sections were counterstained with Mayer's hematoxylin (J.T.Baker, Mallinckrodt Baker, Deventer, the Netherlands) and mounted using xylene-based mounting medium Thermo Scientific Shandon Consul-Mount (Thermo Fisher Scientific Inc.). An irrelevant mouse IgG of the same isotype as the primary antibody dilution served as negative control. Paraffin-embedded placenta tissue samples were used as positive control.
The antibodies used in this study to detect MMP-2 and MMP-9 provided a cytoplasmic pattern of staining. Chromogen containing diaminobenzidine was used to reveal antigen-antibody complexes. Thus immunopositive cells were stained in brown color ranging from light brown to dark brown. Expression of MMPs was graded according to staining intensity.
Two investigators blinded for clinical date and independently of each other carried out grading of expression of MMP-2 and MMP-9 on a semiquantitative scale, in cytoplasm of epithelium/parenchyma and stroma cells. At the first step, the staining intensity of MMP-2 and MMP-9 was graded according to the following criteria: 0, negative staining of cells; 1, weak positivity of cells (cytoplasm stained in light brown color); 2, moderate positivity of cells (cytoplasm stained in brown color); and 3, strong positivity of cells (cytoplasm stained in dark brown color). At the second step, the grades of positively stained epithelial and stroma cells were evaluated as follows: 0, no stained cells; 1, up to 10% of stained cells; 2, from 10% to 50% of stained cells; and 3, more than 50% of stained cells. Whenever there was a disagreement between two observers with a difference in two or more points, the sections were reassessed simultaneously by the same two observers until agreement ranging limit of one point had been achieved. Consequently, difference in agreement in one point was considered as acceptable. Then, the score of MMPs expression was calculated using the following formula.
Score of MMP expression (=grade of MMP staining intensity×grade of stained cells)
Thus, the score of MMPs expression was calculated by multiplied intensity grade (0-3) to proportion grade (0-3), i.e., the maximum score could be 9 for one investigator. Finally, the scores of MMPs expression, both in epithelium and stroma cells estimated by two investigators were summed. Thus, the final score of MMPs expression in both epithelium and stroma cells ranged from 0 to 18 points: 0, no expression; from 1 to 6, low expression; from 7 to 12, moderate expression; and from 13 to 18, high expression.
Internal compatibility between two investigators (interobserver reability) evaluating the expression of the MMPs was substantial - Cohen's kappa 0.65 (P<0.01).
Statistical analysis
For statistical analysis, the program IBM SPSS ver. 19.0 (IBM Co., Armonk, NY, USA) was used. The Mann-Whitney U-test and Kruskal-Wallis test were used for the comparison of two or three groups, respectively. Multiple comparisons among the different groups were conducted with Dunn test. The correlation between MMPs expression and clinical and morphological parameters in patients' groups were evaluated by using Spearman correlation coefficient. Fisher discriminant analysis was applied to determine the system of statistically significant MMPs scores for classifying BVFLs and glottic SCC patients groups. For the evaluation of internal compatibility of investigators, Cohen's kappa coefficient was calculated: 0.21-0.40, fair; 0.41-0.60, moderate; 0.61-0.80, substantial; and >0.80, almost perfect agreements. The significance level of 0.05 was chosen to test statistical hypotheses.
RESULTS
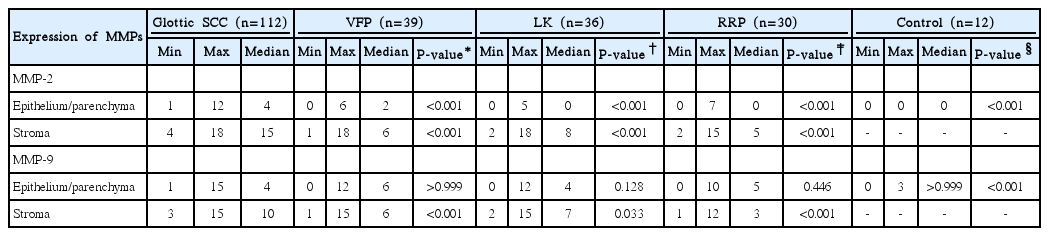
Immunostaining for MMP-2 and MMP-9 was detected in all glottic SCC specimens (100%), both in epithelium/parenchyma (tumor cells) and stroma (Fig. 1). However, in stroma cells, expression of both, MMP-2 (range, 4 to 18 points; median, 15 points) and MMP-9 (range, 3 to 18 points; median, 10 points) was statistically significantly higher (P<0.001), than in epithelium (Table 2). Results of semiquantitative evaluation of MMP-2 and MMP-9 expressions in glottic SCC and BVFL patients' groups are presented in Table 2. It demonstrates a rather sequential increase and statistically significant differences in expression of MMP-2, both in epithelium and stroma, and MMP-9 in stroma among the control group (epithelium), BVFLs and glottic SCC patients with the glottis SCC group having the highest MMPs expression scores (Fig. 2). However, no statistically significant differences were found (P>0.05) among expressions of MMP-9 in epithelium comparing glottic SCC group and VFP, RRP, and LK patients groups, respectively. Only expression of stroma MMP-9 was statistically significantly higher in glottic SCC patients' group comparing with BVFL subgroups. Expressions of epithelium MMP-9 did not differ among the laryngeal pathology groups investigated (Fig. 3).

Immunohistochemical expression of MMP-9, MMP-2 in samples from glottic squamous cell carcinoma (×20). Neoplastic epithelial/parenchyma (white arrow) and stroma cells (black arrow) demonstrate high expression of MMP-9 (A) and high expression of MMP-2 in stroma cells and moderate expression in epithelial/parenchyma cells (B). MMP, matrix metalloproteinase.
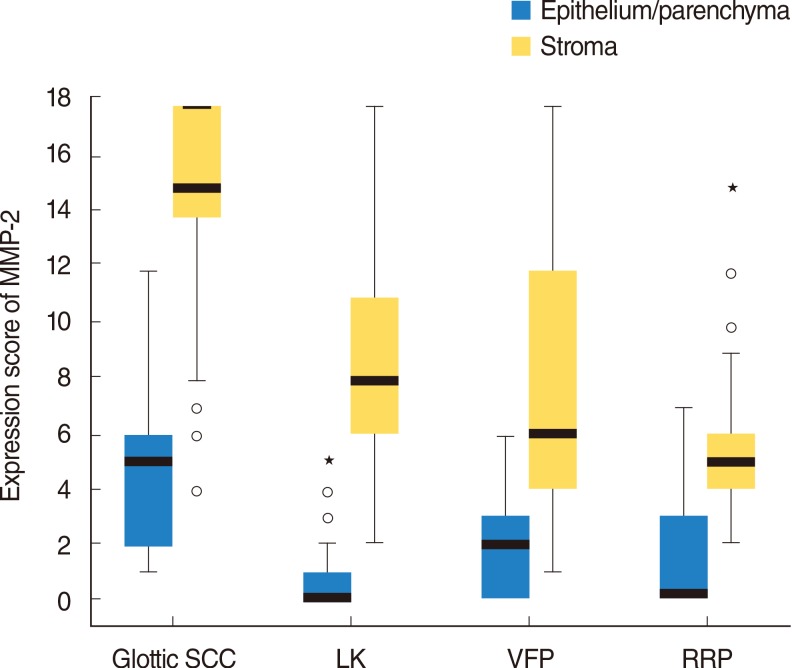
Graphical comparison of MMP-2 expression (boxes represent medians and 25%-75% of cases) among glottic SCC and benign vocal fold lesions patients' groups. MMP, matrix metalloproteinase; SCC, squamous cell carcinoma; LK, laryngeal keratosis; VFP, vocal fold polyps; RRP, recurrent laryngeal papillomas.
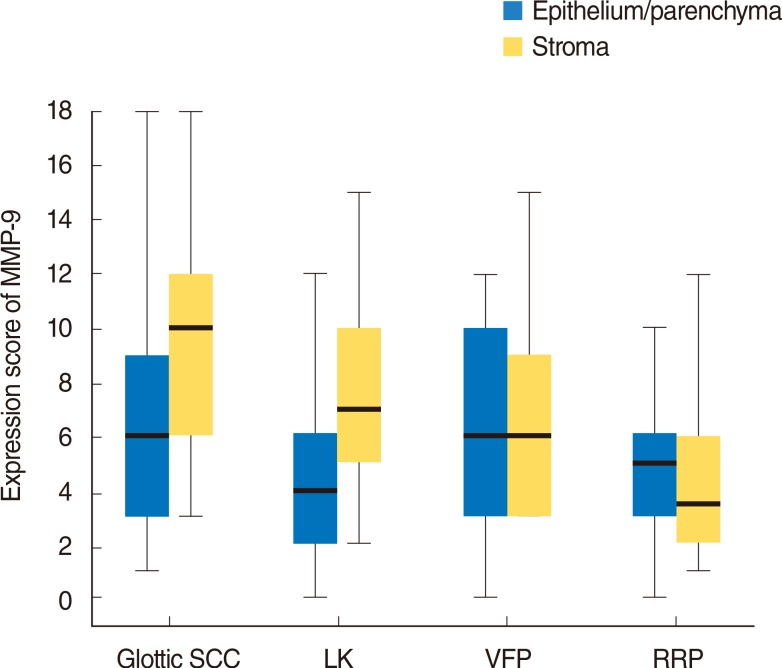
Graphical comparison of MMP-9 expression (boxes represent medians and 25%-75% of cases) among glottic SCC and benign focal fold lesions patients' groups. MMP, matrix metalloproteinase; SCC, squamous cell carcinoma; LK, laryngeal keratosis; VFP, vocal fold polyps; RRP, recurrent laryngeal papillomas.
Results of paired comparison of MMPs expressions between BVFLs groups revealed that in epithelium, expression of both, MMP-2 and MMP-9 was statistically significantly higher in BVFLs groups comparing to the control group (P<0.05). An exception represented only LK group with low expression of MMPs in keratinous epithelium (stroma was not available in control group specimens in our series). However, generally, no statistically significant differences were revealed comparing expressions of both MMP-2 and MMP-9 in groups of BVFL. Again, only exception was represented by statistically significantly lower expression of MMP-2 (P=0.035) in epithelium of LK group comparing to VFP group.
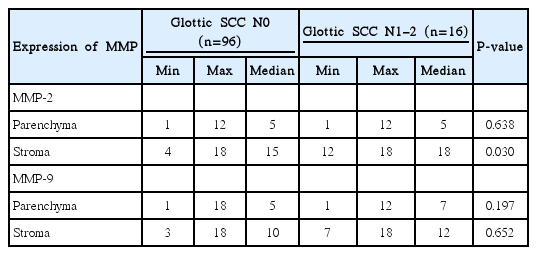
Statistically significant higher expression of epithelium/parenchyma MMP-2 (P<0.001) and stroma MMP-9 (P=0.01) was revealed in the group of moderate/poorly differentiated glottic SCC comparing with well differentiated glottic SCC group (Table 3). The expression only of stroma MMP-2 of the patients' group with glottic SCC and metastases into regional neck lymph nodes (N1-2) was statistically significantly higher than that without metastases (N0) (P=0.03) (Table 4). However, expressions of both, MMP-2 and MMP-9 were not correlated with clinical stage, tumor T value, smoking, alcohol use, age in the glottic SSC patients group.
To determine a statistically significant system of MMPs scores that distinguishes BVFLs patients versus glottic SCC patient group, Fisher discriminant analysis was carried out. After the stepwise procedure of discriminant analysis, only expression of MMP-2 in stroma was selected as a statistically significant parameter for the classification of the study groups. The following two linear discriminant functions fc and fp should be used to classify nonmalignant laryngeal lesions (BVFL)/glottic SCC groups: fc=-2.62+0.53×X; fp=-8.97+1.09×X, where X is the MMP-2 (stroma) value. Consequently, the classification rule is: MMP-2 (stroma)>11.2 points. Therefore, the MMP-2 (stroma) expression score of 11.2 points was determined as the optimum point (limiting value) separating BVFL and glottic SCC patient groups. Parametric values of MMP-2 (stroma) expression that were found to be larger than the limiting value (11.2 points) were considered as pathological (carcinomatous). Sensitivity of MMP-2 (stroma) at that point was 88.3%, specificity 76.7% and odds ratio was 24.8 (95% confidence interval, 11.6-52.8), respectively.
DISCUSSION
The role of MMPs in tumor progression and metastasis has been investigated by numerous studies in a rather large variety of different tumors, including different localizations of SCC [7,16,17]. Generally, the MMPs are considered to be associated with the degradation of the basement membrane collagens [18]. However, the classes of MMP expressed may vary with different cancer types and/or even inside one type of carcinoma (i.e., MMP-2 in laryngeal SCC) [17]. This phenomenon could be a source of some discrepancy of current situation in head and neck squamous cell carcinoma (HNSCC) of different localizations, because the correlation between expression of MMPs and clinical and pathological parameters is still controversial. Therefore, in the present study only glottic SCC cases were included to provide homogeneity of the group investigated and increase reliability of the results.
Another important feature of the present study was a comparative analysis of MMP-2 and MMP-9 expression among BVFL and glottic SCC. Only few studies have been dedicated to investigate the role of MMPs in morphogenesis of benign tumors of larynx [11,15]. Karahan et al. [15] observed significant increases in the expressions of MMP-2, MMP-9 in stromal spindle cells and vascular wall of VFPs compared with normal vocal folds and concluded that gelatinases may play a role in the development of VFPs. One recent study presented data proving the increased expression of MMP-2 and MMP-9 in recurrent laryngeal papillomas [11].
Results of the present study demonstrated that expression of both, MMP-2 and MMP-9 were significantly higher in BVFL groups comparing to the control group. However, generally, no significant differences were revealed among expressions of both MMP-2 and MMP-9 in subgroups of benign laryngeal lesions. Furthermore, our study demonstrated significant differences of MMP-2 and MMP-9 expressions between BVFL and glottic SCC with the latter having higher scores. These data are in concordance with the statement in literature that MMPs (at least MMP-2) are not randomly expressed in HNSCC but rather associated with aggressiveness and metastatic potential of the tumor [17].
Sarioglu et al. [9] demonstrated in their study a sequential rise of the MMP-2 expression with laryngeal carcinomas exhibiting the highest expression; atypical hyperplasias presenting lower expression and carcinoma in situ showing intermediate scores between the two former entities. However, Peschos et al. [11] revealed only one step of increase in the scores of MMP-9 in the progression of dysplasia to invasive carcinoma. Results of the present study indicate that expression of both MMP-2 and MMP-9 were up-regulated early in the development of BVFL, when the benign neoplastic lesion begins and the next determinant step was concerned with the occurrence of malignization. Furthermore, our data show that the presence of MMP secretion in laryngeal tissues may not be of decisive clinical value, because expression of MMPs can be presented both, in cases of benign and malignant tumors. Therefore, comparative quantification of MMPs overexpression in glottic SCC and comparing them with BVF lesions is advocated. Moreover, results of discriminant analysis and limiting value of stroma MMP-2 score that distinguishes BVFL patients versus glottic SCC patients group determined in this study demonstrate prognostic importance of MMP-2 stroma expression in glottic SCC carcinogenesis.
It has been demonstrated in a large variety of studies that MMP-2 and MMP-9 can be produced by keratinocytes, endothelial cells, fibroblasts, osteoblasts, inflammatory, tumor, and other cells, and therefore, due to multiple actions of MMPs regulating a wide variety of cellular functions they play an important role in the physiological and pathological remodeling of tissues [19]. The origin of MMPs secretion in malignant tumors is of particular interest; however, this issue in HNSCC still remains rather controversial. It has not been determined whether MMPs expression originated from tumor cells or peripheral tissues, i.e., tumor-associated stromal cells and inflammatory cells. Several previous studies revealed that the MMPs were more intensively expressed by stromal cells than by tumor/epithelium cells [10,11,12,20,21]. Recent studies have not clarified this problem. Gorogh et al. [17] reported that in their samples of laryngeal SCC MMP-2 and MMP-9 were most prominently expressed within tumor cells. Lee et al. [7] determined that in tonsil SCC MMP-9 expression was detected in tumor cells alone, (which suggests that MMP-9 is produced by tumor cells), however, MMP-2 were expressed in tumor cells alone or in stroma cells. On the contrary, Wittekindt et al. [22], in their study revealed that the localization of MMP-9 expression was not presented in laryngeal SCC cells, but rather in inflammatory stromal cells. Moreover, they demonstrated a significant correlation between stromal MMP-9 expression and the density of blood vessels in stromal septae of laryngeal SCC specimens, thus contributing to angiogenesis.
Results of the present study are in some agreement with the data of the above mentioned investigation, because despite the fact that expression of MMP-9 was revealed in both, epithelium and stroma of all glottic SCC specimens in our series, the expression of MMP-9 in glottic SCC epithelium was statistically significantly lower than in stroma and did not differ significantly from those of BVFL. Finally, data of the present study determined that expression of both, MMP-2 and MMP-9 were significantly higher in glottic SCC stroma cells comparing with glottic SCC epithelium/parenchyma cells. Thus, current evidence indicates, that in glottic SCC tissue MMP-2 and MMP-9 are produced by both, stromal and cancer epithelial/parenchyma cells. These results support data in the literature that EMMPRIN (extracellular matrix metalloproteinase inductor) is supposed to have the potential role of MMP inductor in stromal and tumor cells [23]. On the other hand, the origin of MMP production may depend on the specific type of MMP, which might be characteristic to be preferably produced in certain cell types [17].
Expression of MMPs has already been investigated in numerous studies and a rather large variety of different tumors, including different localizations of SCC. Nevertheless, in HNSCC, the correlations between expression of MMPs and morphological and clinical parameters still remain controversial; therefore prognostic roles of MMPs in laryngeal SCC have not been established [23]. Some of the previous studies have already determined that both, MMP-2 and MMP-9 are associated with lymph node metastasis [7,17,24,25,26] and poor outcome in HNSCC and laryngeal SCC [14,16]. The most recent investigation of Mallis et al. [27] confirmed importance of MMP-2 expression in glottic SCC survival rate. On the other hand, other studies did not find any significant correlation between strong expression of MMP-9 and lymph node metastasis in patients with HNSCC [28,29], neither in laryngeal SCC [22].
In the present study, the expression only of stromal MMP-2 of the patients group with glottic SCC and metastases into regional neck lymph nodes N1-2 was found significantly higher (P=0.030) than that without metastases N0. Expression of epithelial/parenchyma MMP-2 and stromal MMP-9 correlated with histological grading of the glottic SCC, since it was significantly higher in moderate/poorly differentiated glottic SCC. These data are in agreement with results of other studies [22,28,29]. Thus, results of our study confirm an important role of MMP-2 in invasiveness and growth of glottic SCC and support opinion in the literature that in future MMP-2 may be a candidate tumor targeting gene for the gene therapy of laryngeal SCC [30]. Therefore, further investigation in this field on a larger data sample is advocated.
However, no correlations have been revealed between the expressions of MMP-2 and/or MMP-9 and clinical stage, smoking, alcohol use, age and other clinical signs in the glottic SSC patients group in our series.
Some limitations of the present study should be considered. The method of immunohistochemistry utilized in the present study provided data only on total expression of MMPs (active and nonactive forms); therefore data one activity of MMPs are lacking. These factors may confine judging the pathophysiological relevance of increased expression of MMPs in glottic SCC tissue.
In conclusion, the results of the present study indicate that the expression of both MMP-2 and MMP-9 are up-regulated already early in the development of BVFL, when the benign neoplastic lesion occurs and the next determinant step is concerned with the occurrence of malignization. Moreover, results of discriminant analysis and limiting value of MMP-2 revealed in this study demonstrate possible prognostic importance of MMP-2 stroma expression in glottic SCC carcinogenesis. Therefore, it could be presumed that in future assessment and quantification of this marker could be useful in the prediction of neck metastasis in glottic SCC. However, further investigation is necessary.
Notes
No potential conflict of interest relevant to this article was reported.