The Evaluation of Oxidative Stress in the Serum and Tissue Specimens of Patients With Chronic Otitis Media
Article information
Abstract
Objectives
To underline the effect of oxidative stress in chronic otitis media with and without cholesteatoma and to compare the oxidative stress values in the serum and tissue specimens in these two forms.
Methods
The study included a total of 75 individuals, 35 cases with chronic otitis media (COM; 16 females and 19 males) and a healthy control group of 40 cases (20 females and 20 males). The COM patient group was comprised of 18 patients with cholesteatoma and 17 patients without cholesteatoma. All patients underwent mastoidectomy. Serum specimens were taken prior to surgery and diseased tissue specimens from the ear were obtained during surgery from all patients. Only serum specimens were taken from the healthy control cases. The malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GHPx) were measured in the serum and tissue samples of the patient group and in the serum specimens of the control group.
Results
The age ranged from 14 to 48 years in the patient group (mean age, 20.4±12.2 years) and from 19 to 40 years in the control group (mean age, 26.4±4.64 years). When the serum values of all COM patients were compared with those of the control group, in the patient group MDA, which reflects lipid peroxidation, was found to be significantly higher (P<0.01) whereas the antioxidant enzymes SOD, CAT, and GHPx were found to be significantly lower (P<0.01). When the serum and tissue MDA, SOD, CAT, and GHPx values in patients with and without cholesteatoma were compared, no significant difference was found these parameters (P>0.01).
Conclusion
Although oxidative stress plays a role in the pathogenesis of COM with or without cholesteatoma, it may not reflect the severity of the disease. In patients with COM, the evaluation of only serum oxidative stress values without tissue evaluation may be sufficient for assessing oxidative stress.
INTRODUCTION
Chronic otitis media (COM) is the perforation of the ear drum and inflammation of the mucosal lining the hollow space in the middle ear and airy spaces of the temporal bone for more than a three-month period. COM with cholesteatoma is characterized by the presence of an expanding growth consisting of keratinizing squamous epithelim in the middle ear and/or mastoid process [1]. Classically the pathology in COM is limited to the mucoperiosteum.
Any pathology exceeding this limit can result in complications such as osteitis, bone destruction and meningitis [2]. The rate of such complications is over 50%, particularly in COM patients with cholesteatoma [1].
Many studies have investigated the predisposing factors and pathogenesis of COM. Although COM has been described as a multifactorial disesase, its etiopathogenesis has not been fully clarified [1,2,3]. Many factors have been held responsible for the chronicity of inflammation in otitis media such as genetics, eustachian tube malfunction, autoimmunity, infection, osteoclastic activity, cytokines, endotoxins, and lipid peroxidation products related to oxidative stress [4,5,6,7,8,9,10,11]. All these factors also increase free oxygen radicals (FORs) which are accused of being involved in the pathogenesis of various diseases [12].
FORs are essential for immune responses and metabolic activity. Defense cells in the body such as neutrophils, monocytes and macrophages produce FORs as they fight aganst antigens [4]. However, the excess production of FORs causes tissue damage; thus, the normal recovery of the body is hindered and the duration of inflammation is prolonged [13]. Antioxidants are substances that prevent the effect of FORs on cells and provide oxidative balance [12]. They are enzymes that affect and inactivate excessive oxidants [12].
In this study, the pathologic tissue and serum specimens of COM patients, demonstrating mucosal inflammation in middle ear and mastoid cells with and without cholesteatoma, were measured for malondialdehyde (MDA), which indicates lipid peroxidation and the antioxidant enzymes catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GHPx). The serum and tissue results of these patients were compared to those of the control group.
MATERIALS AND METHODS
The study was conducted on COM patients hospitalized in the Clinic of Otorhinolaryngology, at Yuzuncu Yil University Hospital, Van, Turkey. The study included a total of 75 cases, 35 patients with COM (16 females and 19 males) and 40 healthy control cases (20 females and 20 males). In the patient group, 18 had cholesteatoma and 17 did not have cholesteatoma.
The patients were diagnosed as COM with anamnesis and otomicroscopic examination. All patients were examined with pure tone audiometry and computed tomography (CT) of the temporal bone. The patients showing soft tissue density indicating granulation tissue in the mastoid cells and middle ear on CT were selected for the study. None of the patients were receiving regular antioxidant vitamin supplements, such as vitamins E and C. We excluded patients who had acute infections or systemic disease. In addition, the patients were not receiving any drugs, and were not smoking or consuming alcohol.
The control group consisted of 30 healthy subjects that were asymptomatic with an unremarkable medical history and normal physical examination. None of the control subjects were receiving antioxidant vitamin supplementation, such as vitamins E and C. In addition, the subjects were not receiving any drugs, and were not smoking or consuming alcohol.
The study protocol was carried out in accordance with the Helsinki Declaration as revised in 2000. The study protocol was approved by the local ethics committee, and informed consent was obtained from each subject.
Materials collection
Blood samples were obtained in the morning after 12 hours of fasting. Blood samples were collected into empty tubes and immediately stored on ice at 4℃. The serum was then separated from the cells by centrifugation at 3,000 rpm for 10 minutes. Serum samples used for the measurement of MDA, CAT, GHPx, and SOD levels were stored at -40℃ until they were used. All patients underwent mastoidectomy. The tissue specimens were taken intraoperatively from pathological sites in the mastoid cells or middle ear. The specimens were divided into two groups, ones with cholesteatoma and ones without cholesteatoma and then these specimens were frozen in dry tubes at -40℃. No tissue specimens were taken from the control group.
Biochemical analysis
Measurement of lipid peroxidation was measured by estimating MDA levels as described by Yoshioka et al. [14]. The results were expressed as nanomoles per milliliter (nmol/mL) for serum and nanomoles per milligram-tissue (nmol/mg-tissue) for tissue. SOD activity was measured using the Spitz and Oberley's method [15]. In the SOD activity test, 2-(4-iodophenol)-3-(4-nitrophenol)-5-phenyltetrazolium chloride produces a red formazan dye upon reduction with superoxide radicals produced by xanthine oxidase (XO). The rate of the reduction with a superoxide anion is linearly related to the XO activity, and is inhibited by SOD. The SOD activity is determined by the degree of inhibition of this reaction. The results were expressed as units per milliliter (EU/mL) for serum and units per milligram-tissue (nmol/mg-tissue) for tissue.Measurement of CAT activity were measured using H2O2 as a substrate [16]. The degradation of H2O2 was monitored at 240 nm for 5 minutes using a spectrophotometer, and enzyme activity was expressed in units per liter of serum (U/L) for serum and units per gram tissue (U/g-tissue) for tissue.
Measurement of GPHx enzyme activity was performed according to Paglia and Valentine [17]. GHPx enzyme catalyzes oxidation of glutathione. When the oxidized glutathione is reduced, NADPH (nicotinamide adenine dinucleotide phosphate) is oxidized and it is turned into NADP. This change was observed at 340-nm wave and activation of GHPx was measured. The results were expressed as units per liter (EU/mL) for serum and units per gram-tissue (EU/g-tissue) for tissue.
Statistical analysis
All parameters levels both in blood and tissue were compared. The results are expressed as means and standard deviation (mean±SD). Nonparametric continuous variables were compared by Mann-Whitney U-test. Parametric variables were compared using Student t-test. P-value less than 0.001 was considered as statistically significant. Statistical evaluation was carried out with the SPSS ver. 11.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
The age ranged from 14 to 48 years in the patient group (mean age, 20.4±12.2 years) and from 19 to 40 years in the control group (mean age, 26.4±4.64 years). When the serum values of all COM patients with and without cholesteatoma were compared with those of the control group, in the patient group MDA, which reflects lipid peroxidation, was found to be significantly higher (P<0.01) whereas the antioxidant enzymes SOD, CAT, and GHPx were significantly lower (P<0.01) (Tables 1, 2). When the tissue values of the patients (with and without cholesteatoma) were compared with the serum values of the control group (with no tissue specimen), MDA was found to be significantly higher (P<0.01), but the antioxidant enzymes SOD, CAT, and GHPx were significantly lower (P<0.01) both in patients with cholesetatoma (Table 3) and in patients without cholesteatoma (Table 4). On the other hand, when the tissue MDA, SOD, CAT, and GHPx values (Table 5) and serum values (Table 6) of patients with and without cholesteatoma were compared, no significant difference was found among these parameters (P>0.01).

The comparison of serum values of patients with cholesteatoma (P) with those of the control group (C)
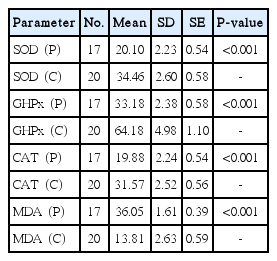
The comparison of serum values of patients without cholesteatoma (P) with those of the control group (C)

The comparison of tissue values of patients without cholesteatoma (P) with serum values of the control group (C)
DISCUSSION
COM, with its treatment stages during the disease process and the complicaitons it can create, retains its importance in the practise of otologists. Neverthless, the etiopathogenesis of the disease has not been fully clarified [1,2,3]. Environmental and immunologic risk factors have been implied in the pathogenesis. Recurrent upper respiratory tract infections, presence of immunosuppressive disease, malnutrition, allergy, nasopharyngeal lymphatic tissue hypertrophy and craniofacial malformations all play a role in the pathogenesis. Many risk factors have been identified for the chronicity of the inflammation [4,5,6,10,11].
As is the case with the pathogenesis of many chronic diseases [12], FORs have recently been held responsible for chronicity of infection and tissue damage in COM [10,11]. A free radical is an atom, molecule or ion that has unpaired valence electrons or an open electron shell, and therefore may be seen as having one or more "dangling" covalent bonds. FORs are produced endogenously during various biochemical reactions in oxygen metabolism [12,18,19]. Excessive production of FORs cause tissue damage by chemically modifiying proteins, carbohydrates, nucleotides and lipids and thus can play a role in the pathogenesis of various diseases [20,21,22].
Normally the tissue damage caused by oxidants in the body is controlled by enzymatic and nonenzymatic antioxidant defense systems [12]. The most important antioxidant enzymes are SOD, GHPx, and CAT; among the nonenzymatic antioxidants are glutathione, tocopherole (vitamin E), ascorbic acid (vitamin C), carotene (vitamin A), albumin, bilirubin and uric acid [12]. Oxidative stress is associated with decreases in antioxidants or with increases in the production of oxidants; this situation, by the peroxidation of phospholipids, causes damage in the vital substances of the body such as lipids, lipoproteins, proteins and DNA [12].
The cell membrane is a critical barrier for free radicals. Free radicals need to pass through this barrier in order to interact with intracellular components. Free radicals initiate lipid peroxidation by eliminating the hydrogen atom from alpha-methylene groups of polyunsaturated fatty acids in the cell membrane [12,19]. At the end of the process, polyunsaturated fatty acids are hydrolysed into biologically active compounds. The most important of these compounds is MDA which reflects lipid peroxidation in the body [14]. The main antioxidants breaking down the lipid peroxidation chain reactions in the cell membrane are SOD, CAT, and GHPx [12,14].
Although FORs have currently been implied in the pathogenesis of many inflammatory diseases, the role of FORs in the pathogenesis of COM has not been fully clarified [23,24]. For this reason, in this study, MDA, which reflects lipid peroxidation, and the antioxidants SOD, CAT, and GHPx were measured in the serum and pathologic tissue specimens of COM patients with cholesteatoma or granulation tissue in the mastoid cells and middle ear.
In inflammation, the production of FORs in the affected area is increased due to the presence of leucocytes [25]. In COM, an inflammation develops in the mucosal lining of spaces as a result of the immune responses to various stimuli. This persistent inflammatory stimulation causes pathologic changes in the tissues and inhibits healthy tissue recovery. Moreover, oxidative stress can damage ciliary structure by damaging cellular DNA and proteins [12,13], thus leading to increased damage in the Eustachian tube and middle ear. In summary, the peroxidation of phospholipids in the cell membranes of the middle ear can prolong the duration of inflammation and thus lead to chronicity. In experimentally induced otitis media with effusion (OME) in rats and guinea pigs it has been demonstrated that FORs was increased in the inflammation site and serum, and the effect of FORs on the chronicity of the disease was highlighted [6,26,27,28]. Khakimov et al. [29] reported that in children with otitis media serum antioxidant enzyme levels were low and that topical alpha-tocopherol, as an antioxidant, shortened the recovery period. Yilmaz et al. [4] demonstrated high levels of serum MDA preoperatively, but low levels of serum SOD, GHPx, retinol, beta-carotene, alpha-tocopherole, lycopene and ascorbic acid in children with ventilation tubes due to adenoidectomy and otitis media with effusion. They showed that antioxidant levels normalized after one month postoperatively and emphasized the requirement of antioxidant therapy in these patients. Garcia Callejo et al. [30] found that the MDA value in the effusion fluid of COM patients with effusion was approximately 10 times higher than that in the middle ear fluid of OME patients. Baysal et al. [11] reported a low total antioxidant capacity and high oxidative stress index in COM patients with and without cholesteatoma.
In this study, the oxidative balance was evaluated in pathologic tissue specimens, and patients with and without cholesteatoma were compared. When the serum values of all COM patients with and without cholesteatoma were compared to those of the control group, the MDA in the patient group was found to be significantly higher (P<0.01) and the antioxidant enzymes SOD, CAT, and GHPx were found to be significantly lower (P<0.01) (Tables 1, 2). When the tissue values of the patients (with and without cholesteatoma) were compared with the serum values of the control group (with no tissue specimen), MDA was found to be significantly higher (P<0.01), but the antioxidant enzymes SOD, CAT and GHPx were significantly lower (P<0.01) both in patients with cholesetatoma (Table 3) and in patients without cholesteatoma (Table 4). On the other hand, when the tissue MDA, SOD, CAT, and GHPx values (Table 5) and serum values (Table 6) of patients with and without cholesteatoma were compared, no significant difference was found in any of the parameters (P>0.01). These findings indicate that evaluating the values of only serum oxidative stress, excluding assessment of tissue values, may suffice for the evaluation of oxidative stress in COM patients. Also, this indicates that the severity of the disease is not parallel to the stress oxidation, in other words, oxidative stress does not reflect the severity of the disease.
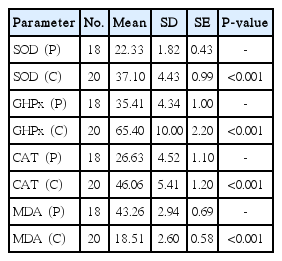
The comparison of tissue values of patients with cholesteatoma (P) with serum values of the control group (C)
In conclusion, oxidative stress due to FORs plays a role in the pathogenesis of both COM with and without cholesteatoma. Assessing the values of only serum oxidative stress, excluding assessment of tissue values, may suffice for the evaluation of oxidative stress in COM patients. However, oxidative stress values may not reflect the severity of the disease.
Notes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.

