Association Between Hearing Impairment and Albuminuria With or Without Diabetes Mellitus
Article information
Abstract
Objectives
Few studies have evaluated the accurate association between hearing loss (HL) and albuminuria in patients with or without diabetes mellitus (DM). The aim of our study was to identify the clinical effects of albuminuria on HL with or without DM.
Methods
This study included 9,762 patients from the Korean National Health and Nutrition Examination Survey between 2011 and 2013. Participants were divided into 4 groups based on DM and urine albumin/creatinine ratio levels: group 1 included participants with neither DM nor albuminuria, group 2 included participants without DM and with albuminuria, group 3 included patients with DM and without albuminuria, and group 4 included patients with both DM and albuminuria. The low- or mid-frequency and high-frequency, and average hearing threshold values were obtained.
Results
There were 7,508, 545, 1,325, and 384 participants in groups 1, 2, 3, and 4, respectively. Univariate and multivariate analyses showed that the 3 hearing thresholds in group 1 were the lowest and those in group 4 were the highest among the 4 groups. No significant differences were observed in those thresholds between groups 2 and 3. Group 4 was associated with HL compared with the other groups, but moderate to severe HL was not associated with DM or albuminuria.
Conclusion
The presence of albuminuria was associated with a modest effect on hearing thresholds regardless of presence of DM.
INTRODUCTION
Diabetes mellitus (DM) and hearing loss (HL) are two common health problems in the general population. Hyperglycemia, advanced glycation end products, and altered intracellular signaling pathways in DM are associated with atherosclerosis or endothelial cell injury, affecting the microangiopathy in various organs and can develop ischemia and hypoxia in end organs [1-3]. The cochlea as a sensitive organ for blood supply is influenced by these pathologic changes. Previous studies showed histopathologic changes such as basement membrane thickening and atrophic changes in the stria vascularis in a DM model [4]. These changes are similar to microangiopathies caused by DM and associated with HL.
Although conflicting results exist, many studies investigated an association between DM and HL [5-8]. Albuminuria has been known as an indicator of endothelial damage in DM patients. Therefore, many researchers think that albuminuria among DM participants will be associated with HL. A population-based longitudinal study showed an association of HL with DM, and HL was more severe in DM patients with severe renal dysfunction than in those without severe renal dysfunction [7]. Shen and Hsieh [9] showed that the amount of albuminuria is associated with hearing impairment in type 2 DM patients. However, albuminuria may be caused by hypertension, age, or obesity, and albuminuria in non-DM participants may also be associated with HL [10]. Previous studies evaluated the effect of DM on HL by comparing DM participants with non-DM participants and the effect of albuminuria on HL among the DM patients, but these studies did not consider the effect of albuminuria in non-DM participants. Therefore, previous reports did not evaluate the accurate association between HL and albuminuria in DM patients or non-DM participants. The aim of our study was to identify the clinical effects of albuminuria on HL with or without DM.
MATERIALS AND METHODS
Study population
This study was based on the Korean National Health and Nutrition Examination Survey between 2011 and 2013 (n=24,594). A total of 14,832 participants were excluded for the following reasons: no data regarding pure tone audiometry (n=9,033); no clinical data (n=2,057); and <40 years old (n=3,742). Finally, 9,762 patients were enrolled in the present study. Local ethical committee approval was obtained for our study.
Study variables
We have reviewed the following data: age, sex, body mass index (BMI, kg/m2), alcohol and smoking behaviors, history of exposure to occupational or explosive noise, estimated glomerular filtration rate (eGFR, mL/min/1.73 m2), glycated hemoglobin (HbA1c) level (%), blood pressure (mmHg), urine albumin/creatinine ratio (UACR, mg/g), and lipid profiles, and hearing thresholds.
Urinary albumin levels were obtained from random samples and calculated using a turbidimetric immunoassay. Urinary creatinine levels were calculated using a colorimetric method. Both urinary albumin and creatinine levels were calculated in the same laboratory for all measurements (Hitachi Automatic Analyzer 7600, Hitachi, Tokyo, Japan). The inter-assay coefficient of variation, calculated from all laboratory work, was low (<3.1%). UACR was calculated as the urine albumin to creatinine ratio in milligrams per gram of creatinine (mg/g). In our study, albuminuria was defined as levels of UACR ≥30 mg/g. DM was defined as having a fasting glucose level of ≥126 mg/dL, HbA1c ≥ 6.5%, or a self-reported history of DM diagnosis. Participants were divided into 4 groups based on DM and UACR levels: group 1 included participants with neither DM nor albuminuria, group 2 included participants without DM and with albuminuria, group 3 included participants with DM and without albuminuria, and group 4 included participants with both DM and albuminuria. The eGFR was calculated using the 4-variable modification of diet in renal disease (MDRD) equation [11]. Hypertension (HTN) was defined as having systolic blood pressure (SBP) ≥140 mmHg, diastolic blood pressure (DBP) ≥90 mmHg, use of anti-hypertension drugs, or self-reported history of HTN. Smoking behaviors were divided into non-smoker, exsmoker, or current smoker. Alcohol intake was classified by the Korean version of standard drinking, which is based on the World Health Organization (WHO) [12,13]. We divided into 3 groups as follows: abstinence (not having had an alcoholic drink within the last year), moderate drinking (0.10–19.99 and 0.10– 39.99 g alcohol/day for women and men, respectively), and heavy drinking (≥20 and ≥40 g alcohol/day for women and men, respectively). History of exposure to explosives or occupational noise was defined as positive or negative, as previously described [14].
The air conduction hearing thresholds were measured at six frequencies (0.5, 1, 2, 3, 4, and 6 kHz). No patients had been receiving ototoxic medications or had ear diseases such as otitis media. For both ears of each participant, the low- or mid-frequency (low/mid-frequency) and high frequency (high-frequency) values were calculated by the pure tone averages at 0.5, 1, and 2 kHz and at 3, 4, and 6 kHz, respectively. The average hearing threshold (AHT) was obtained by the pure tone average at 0.5, 1, 2, and 3 kHz. In our study, HL was defined as AHT >25 dB. Mild and moderate to severe HL were defined as 25 dB<AHT≤40 dB and AHT>40 dB, respectively.
Statistical analyses
IBM SPSS ver. 21 (IBM Co., Armonk, NY, USA) was used for statistical analyses. Categorical data were expressed as number (%). Continuous data were expressed as mean and standard deviation. Hearing thresholds were expressed as mean and standard error. The categorical data were analyzed using Pearson chi-square test or Fisher exact test. Continuous variables were analyzed using one-way analysis of variance. Multivariate analyses using analyses of covariance were used to evaluate the differences in hearing thresholds among groups. Binomial or multinomial logistic regression analyses were used to calculate odds ratios (95% confidence intervals [CIs]) to determine the relationships between groups and HL. Multivariate analysis was adjusted for gender, age, high-density lipoprotein (HDL) cholesterol levels, triglyceride levels, eGFR, SBP, DBP, BMI, alcohol consumption, smoking behavior, HbA1c levels, and exposure to explosives or occupational noise. A P-value <0.05 was defined as having statistical significance.
RESULTS
Clinical characteristics of the participants
There were 7,508, 545, 1,325, and 384 participants in groups 1, 2, 3, and 4, respectively (Table 1). The mean age and proportion of men in group 4 were greater than the other groups. A greater proportion of participants in group 4 had a history of occupational or explosive noise compared with the other groups. The eGFR decreased going from group 1 to group 4. The UACRs were 5.0±5.5 (range, 0.0 to 29.8), 146.1±253.3 (range, 30.1 to 2,541.0), 8.4±7.4 (range, 0.1 to 29.8), and 285.7±664.0 (range, 30.2 to 6,568.3) mg/g in groups 1, 2, 3, and 4, respectively. Groups 2 and 4 with albuminuria had higher SBP than the other groups. HDL cholesterol was lower in the groups with DM than in the groups without DM. There were 2,653 (35.3%), 373 (68.4%), 801 (60.5%), and 289 (75.5%) participants with HTN in groups 1, 2, 3, and 4, respectively.
Comparison of hearing thresholds according to DM or albuminuria
Univariate analysis showed that low/mid-frequency values in groups 1, 2, 3, and 4 were 17.6±0.2, 22.7±0.7, 22.4±0.4, and 25.5±0.9 dB, respectively (Fig. 1). The high-frequency values in groups 1, 2, 3, and 4 were 32.2±0.2, 39.2±1.0, 40.2±0.6, and 45.2±1.1 dB, respectively. The AHT values in groups 1, 2, 3, and 4 were 19.5±0.2, 24.8±0.7, 24.9±0.4, and 28.5±0.9 dB, respectively. The 3 hearing thresholds in group 1 were lower than those in the other groups, and those in group 4 were higher than those in groups 2 and 3. However, there were no significant differences in the 3 hearing thresholds between groups 2 and 3.

Hearing thresholds according to diabetes mellitus and albuminuria. The multivariate analysis was adjusted for age, sex, high-density lipoprotein cholesterol levels, triglyceride levels, estimated glomerular filtration rate, systolic blood pressure, diastolic blood pressure, body mass index, alcohol consumption, smoking behavior, glycated hemoglobin levels, and exposure to explosives or occupational noise (*P<0.05 compared with group 1; †P<0.05 compared with groups 1, 2, and 3). The data are expressed as mean and standard error. AHT, average hearing threshold.
Multivariate analysis showed that the low/mid-frequency values in groups 1, 2, 3, and 4 were 18.4±0.2, 19.8±0.5, 19.7±0.4, and 21.7±0.7 dB, respectively. The high-frequency values in groups 1, 2, 3, and 4 were 33.8±0.2, 35.3±0.7, 35.2±0.6, and 37.9±1.0 dB, respectively. The AHT values in groups 1, 2, 3, and 4 were 20.5±0.2, 21.7±0.5, 21.7±0.4, and 24.0±0.7 dB, respectively. Multivariate analysis showed trends that were similar to the univariate analysis results.
Association between HL and albuminuria with or without DM
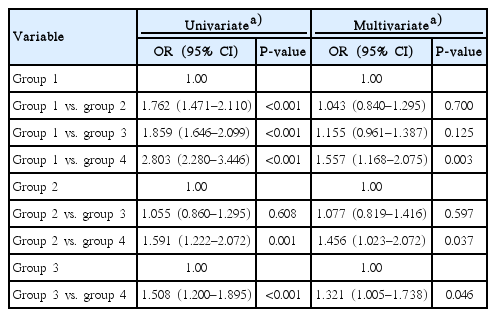
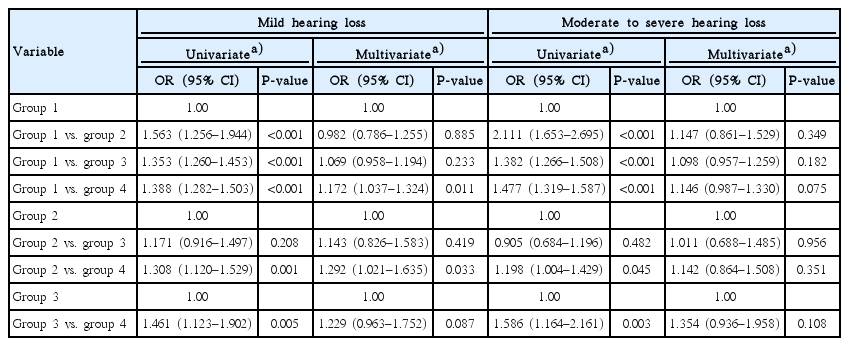
The numbers of participants with mild HL were 1,247 (16.6%), 118 (21.7%), 329 (24.8%), and 116 (30.2%) in groups 1, 2, 3, and 4, respectively (P<0.001). The numbers of participants with moderate to severe HL were 712 (9.5%), 91 (16.7%), 196 (14.8%), and 75 (19.5%) in groups 1, 2, 3, and 4, respectively (P<0.001). Univariate analysis showed an association between HL and the presence of DM or albuminuria (Table 2). There was no significant difference in HL between groups 2 and 3. Multivariate analysis showed significant associations only between group 4 and the other groups. Multinomial logistic regression analysis showed an association in mild HL between group 4 and group 1 or group 2 (Table 3). Compared with group 1, group 4 had a 1.172-fold (95% CI, 1.037 to 1.324; P=0.011) higher risk for mild HL. Compared with group 2, group 4 had a 1.292-fold (95% CI, 1.021 to 1.635; P=0.033) higher risk for mild HL. There were no significant differences in moderate to severe HL among the 4 groups.
DISCUSSION
Univariate and multivariate analyses showed that the 3 hearing thresholds in group 1 were the lowest and those in group 4 were the highest among the 4 groups. There were no significant differences in those thresholds between groups 2 and 3. Group 4 was associated with HL compared with the other groups, but moderate to severe HL was not associated with DM or albuminuria.
Regarding histopathologic changes developed in a DM model, the association between DM and HL may be legitimate. However, there are conflicting results for associations between the 2 variables. Previous cross-sectional studies showed that DM was linked with prevalent HL, but longitudinal studies did not show these associations [7,8,15,16]. These conflicting results may be related to confounders such as duration of DM, onset of HL, small cohorts, and other comorbidities [17]. The association between the 2 variables is not conclusive, and further investigations are needed to identity the effect of DM on HL. The present study enrolled a large population, and the results can help to evaluate the relationship of the 2 variables.
Albuminuria has been known as an early indicator for microangiopathies in DM. Development of microangiopathy may be related to similar pathologic changes in the cochlea, which can manifest clinically as HL. Dalton et al. [7] showed an association between DM nephropathy and HL, but in their study, DM nephropathy was defined as heavy proteinuria, azotemia, or end-stage renal disease. Kakarlapudi et al. [18] showed an association between serum creatinine levels and HL in DM. The previous 2 studies revealed that advanced DM nephropathy is associated with HL in DM. Shen and Hsieh [9] showed an association between albuminuria and HL in DM. However, their study enrolled only 68 DM participants and did not compare DM participants with non-DM participants. The present study divided the study population into various groups according to the presence of DM or albuminuria as an early DM nephropathy. The present study revealed that DM regardless of albuminuria was associated with high hearing thresholds compared with non-DM without albuminuria. However, no significant differences were observed in the three hearing thresholds between DM patients without albuminuria and non-DM patients with albuminuria. DM with albuminuria was associated with HL compared with the other groups, but these associations were observed only for mild HL.
Albuminuria in non-DM participants can be caused by obesity, HTN, or hyperuricemia and is associated with mortality or morbidities in the general population [19-23]. A large population study showed that the prevalence of albuminuria is approximately 14.5% in the non-DM hypertensive population and 5.1% in the non-DM non-hypertensive population [24]. Albuminuria in non-DM patients is related to endothelial injury or chronic kidney disease, which can induce HL. However, there are few investigations of the association between HL and albuminuria in participants without DM. Our data shows a modest effect of albuminuria in non-DM participants. There was a significant increase in the 3 hearing thresholds between the presence and absence of albuminuria in non-DM participants, but the difference was small. There was no significant difference in HL, as a categorical criterion, between the 2 groups. In addition, the presence of albuminuria in DM or non-DM participants was associated with high hearing thresholds compared with the absence of albuminuria in the same DM or non-DM participants. These reveal that albuminuria may have an additive effect on hearing thresholds in both DM and non-DM participants.
Our study showed both univariate and multivariate analyses, but statistical significances were different. A multivariate analysis would be more valuable. Our study is a retrospective study and we did not make the baseline characteristics coincide as shown Table 1. Our data showed significant differences in age, sex, and various metabolic parameters among the groups and these would be confounding factors for the evaluation of hearing impairments. Multivariate analyses adjusting for possible confounding factors would be more valuable rather than only univariate analyses. In our study, results from multivariate analyses were less statistically significant than those from univariate analyses.
The study has a number of limitations. First, this study design was cross-sectional nature, meaning we could not establish causality. Second, the participants in our study included an ethnically homogeneous population. Third, this study did not include the sensitive components regarding hearing problems such as speech discrimination. Data for 250 Hz were also not included. Although data for 250 Hz can be more accurate for evaluating low frequency hearing thresholds, previous studies have defined low frequency hearing thresholds without including data for 250 Hz [25-29]. In addition, these studies have defined low/mid-frequency using the pure tone averages at 0.5, 1, and 2 kHz [25-28]. In addition, this study did not evaluate different DM types such as type 1 or type 2 DM. In the present study, there were only 5 DM participants diagnosed at age 30 years or younger in group 2 and 5 in group 4. Therefore, most DM participants in our cohort had be type 2 DM.
In conclusion, the presence of albuminuria was associated with a modest effect on hearing thresholds regardless of presence of DM. DM without albuminuria was also associated with high hearing thresholds compared with the normal population, but the effect was modest. DM participants with albuminuria had significantly more HL compared with the other participants. Therefore, participants with albuminuria should be closely monitored for hearing impairment.
HIGHLIGHTS
▪ Albuminuria has been known as an indicator of endothelial damge in diabetes mellitus (DM) patients.
▪ The presence of albuminuria was associated with a modest effect on hearing thresholds regardless of presence of DM (P<0.05).
▪ DM without albuminuria was also associated with high hearing thresholds compared with the normal population, but the effect was modest (P<0.05).
▪ DM participants with albuminuria has significantly more hearing loss compared with the other patients (P<0.05).
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2014R1A1A3049993).