Guidelines for the Surgical Management of Laryngeal Cancer: Korean Society of Thyroid-Head and Neck Surgery
Article information
Abstract
Korean Society of Thyroid-Head and Neck Surgery appointed a Task Force to develop clinical practice guidelines for the surgical treatment of laryngeal cancer. This Task Force conducted a systematic search of the EMBASE, MEDLINE, Cochrane Library, and KoreaMed databases to identify relevant articles, using search terms selected according to the key questions. Evidence-based recommendations were then created on the basis of these articles. An external expert review and Delphi questionnaire were applied to reach consensus regarding the recommendations. The resulting guidelines focus on the surgical treatment of laryngeal cancer with the assumption that surgery is the selected treatment modality after a multidisciplinary discussion in any context. These guidelines do not, therefore, address non-surgical treatment such as radiation therapy or chemotherapy. The committee developed 62 evidence-based recommendations in 32 categories intended to assist clinicians during management of patients with laryngeal cancer and patients with laryngeal cancer, and counselors and health policy-makers.
INTRODUCTION
According to statistical data obtained from the Korean National Cancer Center in 2013, the prevalence of laryngeal cancer was 72.7 per 100,000 individuals, and after thyroid cancer, laryngeal cancer was the second-most commonly encountered type of head and neck cancer. Treatment plans for laryngeal cancer have been well documented by the National Comprehensive Cancer Network and National Cancer Institute. Previously, most surgical treatments for laryngeal cancer comprised total laryngectomy, and the resulting loss of voice significantly impacted patients’ postoperative quality of life. After the trials by the Veterans’ Affairs Administration [1] and Radiation Therapy Oncology Group (RTOG 91-11) [2], non-surgical organ-preserving treatments have become standard therapies for laryngeal cancer. Surgery, however, still plays a role in the treatment of laryngeal cancer. In particular, a recently introduced technique broadened the indications for transoral surgery, and many early laryngeal cancers that previously would have been treated with open partial laryngectomy can now be treated with transoral surgery. Furthermore, supracricoid laryngectomy (SCL) has yielded excellent functional and oncological outcomes, even in selected advanced cases. Surgical techniques have changed over time, parallel with the continuous development of new techniques and devices. As a result, Korean Society of Thyroid-Head and Neck Surgery (KSTHNS) sought to develop guidelines for the surgical treatment of laryngeal cancer with the intent to facilitate evidence-based decision making in this era of rapidly changing treatment trends. These guidelines are not intended to replace clinical judgements and should not be used to solve medico-legal issues. In other words, these guidelines should be used only as an adjunct to clinical judgement.
Target population
These guidelines are intended for patients with suspected or diagnosed laryngeal cancer. These guidelines primarily target patients who agree to undergo surgery after a discussion about multimodal treatments. The guidelines suggest an appropriate diagnostic workup for patients with laryngeal cancer and especially focus on the preoperative evaluation. Separate recommendations for initial surgical treatment are described for glottic and supraglottic carcinomas. Information about postoperative follow-up, complications, and management of recurrences is also included.
Intended users
These guidelines are mainly intended for head and neck surgeons who treat patients with laryngeal cancer. Furthermore, detailed information about surgical treatments will promote the understanding of surgical treatments for laryngeal cancer by other clinicians who work within multimodal team settings, including medical and radiation oncologists, rehabilitation department workers, nurses, patients, health policy makers, and counselors who provide patient support.
MATERIALS AND METHODS
Organization of the committee
The chairman of this Task Force (SYK) for the development of guidelines for the surgical treatment of laryngeal cancer was recommended by KSTHNS. The chairman led a committee that included two secretaries (SHA, HJH) and 12 members (KHK, JLR, JR, JHP, SKB, GHL, SYL, JCL, MKC, YHJ, YBJ, and JHH). The committee initially met in May 2015 and held a total of 22 meetings. During the sixth meeting, a 14-member practice committee (MK, YMP, CMS, SCS, CHR, DYL, YCL, JWC, HMJ, JKC, WC, BJC, IJC, and HGC) was organized to perform the literature search and review. The guideline committee had complete editorial independence from KSTHNS.
Selection of key questions
The goal of this project was the development of comprehensive guidelines regarding surgical treatment, including preoperative and postoperative evaluation. Accordingly, we divided topics into four categories: preoperative evaluation, surgery for glottic cancer, surgery for supraglottic cancer, and postoperative follow-up and management of complications and recurrences. A key question to be addressed was formulated for each category. The selected key questions are listed in Table 1.
Literature search and quality assessment
In the seventh and eighth committee meetings, held on November 3 and December 8, 2015, the committee reached a consensus about the keywords that would be used in the literature search for a systematic review of the key questions. This literature search was performed on January 16, 2016. The MEDLINE, EMBASE, Cochrane Library, and KoreaMed databases were searched for all available papers using the same keywords. The results of these searches were saved in Endnote X6 (Thomson Reuters, New York, NY, USA), and duplicates were removed. The inclusion criteria were as follows: (1) a human study population; (2) publication type of article, review, or article in press; and (3) English or Korean language text. Following a title review, irrelevant articles were excluded; the remaining selected articles were reviewed independently by two committee members who determined the exclusion or inclusion of papers. Case report, commentaries, and older publications for which the full text was not available were excluded. The keywords used for the selected key questions, number of retrieved papers, and search results are listed in Supplementary Table 1.
Qualification of literature and grades of recommendations and evidence levels
The abstracts and texts of papers selected using the above-described methods were reviewed. The literature quality was classified as follows: (1) randomized controlled trials (RCTs) or well-designed systematic reviews or meta-analyses; (2) non-RCTs; (3) high-quality case-control or cohort studies, including multicenter studies; (4) case reports or clinical studies without control groups; and (5) expert opinions. As it is nearly impossible to obtain high-quality papers (e.g., those describing well-designed RCTs) in the field of surgical management, we classified well-designed meta-analyses and systematic reviews as high-quality evidence. RoBANS (Risk of Bias Assessment Tool for Nonrandomized Study) [3] was used for the quality assessment of non-RCTs and observational studies, and AMSTAR (A Measurement Tool to Assess the Methodological Quality of Systematic Reviews) [4] was used for the assessment of systematic reviews and meta-analyses.
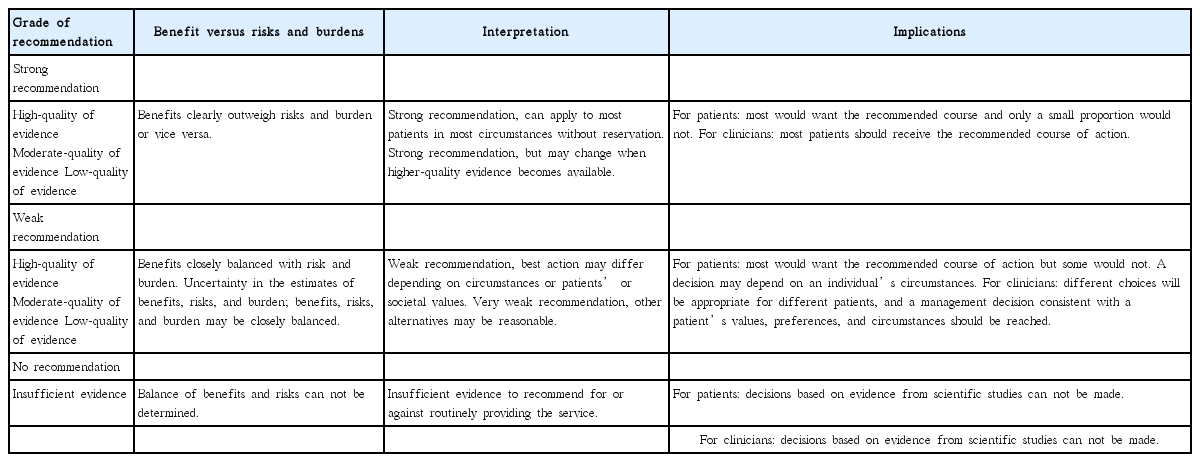
These guidelines adopted the American College of Physicians (ACP) grading system [5]. This system uses only two basic levels of recommendation, strong and weak; accordingly, it has the advantages of simplicity and easy interpretation by clinicians or patients [6]. The level of evidence was classified as high-quality, moderate-quality, or low-quality evidence (Table 2). For controversial issues with inconsistent data, a decision of “no recommendation” was made because of insufficient evidence. “No recommendation” does not mean that the committee is against the action; it merely indicates that the committee cannot decide for or against the issue. This interpretation of the grading system, which follows the guidelines provided by the ACP, is summarized in Table 3. The level of evidence was reviewed, and during the 18th committee meeting, a consensus was reached on the basis of the references used to make each recommendation (Supplementary Table 2). The levels of recommendations, moreover, were rated in consideration of the current situation in Korea.
Consensus regarding recommendations and manuscript development
The recommendations were sent via e-mail to senior head and neck surgeons in order to elicit expert opinions and seven surgeons suggested the opinions. The guidelines were then revised based on the comments received from this expert consultation. After finalizing the recommendations, the Delphi panels were composed of experts with more than 10 years of experience in the KSTHNS to ensure that the panel would be representative of the group of head and neck surgeons. The panel comprised 50 head and neck surgeons to whom the Delphi questionnaire and draft of the guidelines were sent via e-mail. The level of agreement was graded using the following Likert scale: (1) fully agree; (2) agree; (3) neither agree nor disagree; (4) disagree; and (5) totally disagree. If more than two-thirds of the panel members responded with 1 or 2, the recommendation was ultimately accepted. After the first round of the Delphi questionnaire, 36 surgeons replied the first Delphi questionnaire and the response rate was 72%. The consensus was achieved for 58 of the 63 recommendations (92.1%) (Supplementary Table 3). The remaining five recommendations were revised according to the Delphi responses and did second round Delphi questionnaire. Forty surgeons replied the second Delphi questionnaire and the response rate was 80%. Four out of five recommendations could get agree but one is failed to achieve more than 2/3 agree of panels in the second round and this recommendation was removed from manuscript (Supplementary Table 3).
Limitations of guideline development
As the guidelines mainly focused on surgical treatment, very few well-designed studies with high-quality evidence were available. Therefore, many recommendations were based on expert opinions or case series from retrospective studies. In addition, these guidelines were developed for head and neck surgeons who intend to administer surgical treatment for laryngeal cancer, and readers should not therefore interpret these guidelines to favor surgical over non-surgical treatment. Another limitation was our inability to make guidelines that would best address the situation in Korea, as the amount of data from Korea was not sufficient to make nation-specific recommendations. Therefore, a multicenter approach to the publication of Korean treatment data is needed.
Plan for release and update of guidelines
The guidelines will be published in an open access journal to allow better access to the contents, and the publication of these guidelines will be advertised on the homepage of KSTHNS. Reprints of these guidelines will be distributed to otolaryngology head and neck surgery clinics that provide treatment to patients with laryngeal cancer.
The guideline development task force will be maintained as a special committee in KSTHNS, and the guidelines will be revised and updated every 3 to 5 years to incorporate new clinical data and advances in surgical and diagnostic techniques.
GUIDELINES FOR SURGICAL TREATMENT OF LARYNGEAL CANCER
The oncological outcomes of radiation therapy or concurrent chemoradiotherapy are comparable to those of surgery, and the former modalities may provide superior results to surgery in terms of the quality of life. However, surgery is still preferred for very advanced T4 cases. Accordingly, a patient should be offered all relevant information about the different treatment modalities. We begin with the assumption that a comprehensive discussion of the pros and cons of non-surgical versus surgical strategies has been completed and surgery has been determined as the treatment modality in each situation. Therefore, these guidelines address issues related to the decision for primary head and neck surgery (Table 4).
Recommendation 1
A multidisciplinary team approach is recommended for decision-making regarding the treatment of patients with laryngeal cancer, and patients should be provided sufficient information about the roles of chemotherapy, radiation therapy, and surgery (strong recommendation, high-quality evidence).
A. Diagnosis and work up of laryngeal cancer
A1. What is the role of a laryngoscopic examination and voice analysis in the diagnosis of laryngeal cancer?
A clinical diagnosis of laryngeal cancer can usually be made on the basis of the laryngeal images obtained during an examination. Diagnostic tumor confirmation is performed through a careful examination, including a physical examination, flexible laryngoscopy, endoscopic examination under general or local anesthesia, biopsy, and radiologic evaluation. An instrumentbased laryngeal evaluation could lead to the early detection of laryngeal cancer. Flexible fiberscopic laryngoscopy permits image and video documentation, as well as evaluations during actions such as Valsalva’s maneuver, phonating, coughing, and swallowing. Compared with conventional laryngoscopy, laryngeal videostroboscopy is a better technique for the documentation of tiny lesions on the vocal folds and assessments of mucosal waves before and after surgery.
Recommendation 2
(A) A laryngoscopic examination of patients with hoarseness is an essential step in the early diagnosis of laryngeal cancer (strong recommendation, low-quality evidence).
(B) Stroboscopic examination can be used to evaluate suspicious lesions on the vocal folds (weak recommendation, low-quality evidence).
(C) Narrow band imaging (NBI) and indirect autofluorescence endoscopy may be useful for conducting laryngeal cancer examinations (weak recommendation, low-quality evidence).
Visualization of the larynx is an essential component of the initial evaluation of a patient with an early glottic lesion. Laryngoscopy and videostroboscopy are the primary diagnostic instruments used to assess glottic lesions. Physicians routinely use rigid telescopic laryngoscopy with stroboscopy to diagnose vocal fold pathology and assess the vibratory function of the glottis. It has become standard practice in many institutions to make a permanent video record of the appearances of all laryngeal cancers via magnified rigid telescopy or fiberoptic endoscopy. The office-based use of flexible laryngoscopy has augmented the abilities of clinicians to conduct laryngeal assessments in individuals who may not tolerate rigid laryngoscopy and mirror examination, or in those requiring enhanced visualization. Flexible laryngoscopy allows an examination that is less operator- and/or patient-dependent than mirror laryngoscopy, provides a magnified view of the larynx, permits examination archiving, and is well tolerated [7]. Additionally, stroboscopy, which facilitates the assessment of vocal fold vibratory capabilities, was found to be critical in the diagnosis of voice disorders and has altered treatment decisions in otolaryngology practice [8,9]. Particular diagnoses were more consistently identified; for example, cancer was much more accurately identified using laryngoscopy (100%) and stroboscopy (100%) when compared with history and physical examination alone (33%) [10]. In dysphonic patients, laryngeal visualization (flexible laryngoscopy and stroboscopy) should be performed, and the lack of accuracy of a diagnosis based solely on history and physical examination has been confirmed in patients with hoarseness [10]. Routine videostroboscopy can be an important, simple, noninvasive tool that allows a proper and accurate evaluation of glottic leukoplakia in a single procedure [11]. The modern use of microlaryngology has improved the diagnosis and treatment of early and advanced glottic lesions [12].
However, laryngeal diagnosis associated with videolaryngostroboscopy still provides odds for patients with multiple diagnoses, vocal fold paralysis, and paresis, followed by those with nonspecific dysphonia, benign vocal fold/laryngeal pathology, acute and chronic laryngitis, and laryngeal cancer [13]. Accordingly, a more accurate diagnostic method, such as NBI, is needed. The ability of NBI to detect changes in the mucosal microvasculature can be useful for distinguishing nonmalignant from malignant lesions [9,14]. NBI has a reported sensitivity of 93.2% for the detection of laryngeal cancer, in comparison with 68.5% for white light endoscopy [12]. The widespread use of indirect autofluorescence endoscopy during follow-up to identify synchronous/metachronous second tumors of the upper aerodigestive tract may be warranted [15].
For laryngeal glottic lesions, a microscope is used to view the larynx through a transorally placed laryngoscope. This precise microsurgical method is used for the biopsy and staging of early and advanced malignant tumors of the glottis [16].
The enhanced color images provided by electronic videoendoscopic systems are superior in both quality and resolution to those obtained by conventional flexible fiberoptic endoscopy with a video camera. This system is expected to be a valuable tool for the diagnosis of laryngeal lesions [17].
A2. What are the roles of computed tomography (CT) and magnetic resonance (MR) for the diagnosis of laryngeal cancer?
Recommendation 3
Preoperative cross-sectional imaging studies (CT, MR) with contrast are recommended for the staging and pretreatment assessment of laryngeal cancer (strong recommendation, moderate-quality evidence).
Clinical examinations of laryngeal cancer, such as endoscopy and biopsy, can fail to detect pathologic involvement of the deep laryngeal space, whereas cross-sectional imaging (CT, MR) allows a more accurate assessment of the tumor depth and extent. A precise assessment of the tumor extent toward the pre-epiglottic and paraglottic spaces and the detection of cartilage invasion play vital roles in treatment planning for laryngeal cancer. CT was found to be highly accurate for the staging of transglottic (88%) and supraglottic involvement (68%) when compared with the pathologic findings [18]. Zbaren et al. [19] reported that a combination of clinical/endoscopic evaluation and additional imaging workup (CT, MR) provided significantly superior staging accuracy (80% vs. 87.5%). T1-weighted MR images yielded a specificity of 84% and accuracy of 90% for the prediction of invasion of the pre-epiglottic space [20]. In the paraglottic space, the sensitivity of magnetic resonance imaging (MRI) ranges from 93% to 95%; however, the specificity is only 50% to 76%. In a study of 45 laryngeal carcinomas, CT assessment of the paraglottic and the pre-epiglottic space yielded accuracy rates of 88% and 95%, respectively, whereas MR assessment yielded correct interpretations in 90% and 93% of cases, respectively [21]. In a recent prospective study of MR images, the sensitivity rates for infiltration of the pre-epiglottic and paraglottic spaces were 89% and 67%, respectively; the corresponding specificity rates were 97% and 50%, respectively [22]. Furthermore, Banko et al. [23] demonstrated an accuracy rate of 100% in the MR-based assessment of anterior commissure involvement.
Although involvement of the inner thyroid cartilage cortex does not change the tumor stage, it does influence the treatment modality. Gross thyroid cartilage invasion can be detected with CT. According to previous reports, CT findings of cartilage invasion include sclerosis, erosion, lysis, and frank extralaryngeal tumor spread [24,25]. However, CT often fails to diagnose early cartilage invasion because of variability in the laryngeal cartilage ossification pattern [26]. Therefore, the CT-based detection of thyroid cartilage invasion mainly depends on the diagnostic CT criteria. Becker et al. [27] reported that the selection of an appropriate combination of CT criteria yielded an overall sensitivity of 91% and overall specificity of 79%. In a series of 107 consecutive previously untreated laryngectomy specimens, the positive predictive values (PPVs) of CT for thyroid cartilage penetration and extralaryngeal spread were 74% and 81% [28]. In a recent study, Xia et al. [29] reported PPVs of 79% to 80% and negative predictive values (NPVs) of 93% to 100% for thyroid cartilage invasion with CT. Although moderate PPVs imply a risk of overtreatment, CT may be considered as an excellent tool to exclude cartilage invasion prior to treatment [30]. MR is widely considered superior for the assessment of muscle and cartilage invasion, with reported sensitivities of 89% to 94% and specificities of 74% to 88% for thyroid cartilage invasion in laryngeal cancer [31]. In a series of 23 patients who underwent laryngectomy, the sensitivity, specificity, efficiency, PPV, and NPV of MR for inner thyroid lamina invasion were 93%, 82%, 88%, 88%, and 90%, respectively; the corresponding values for outer thyroid lamina invasion were uniformly the same (85%) for all parameters [15,32]. However, other studies reported that MR has a relatively low PPV (68% to 71%) for the detection of thyroid cartilage invasion [19,33]. In a prospective study of 53 patients with carcinoma of the larynx or pyriform sinus who underwent CT and MR imaging before total or partial laryngectomy, MR was more sensitive (89% vs. 66%, P=0.001) but less specific than CT (84% vs. 94%, P=0.004) [33]. Therefore, false positive results are inevitable with both imaging tools, and this phenomenon is reflective of the shared underlying pathologic process, namely reactive inflammation, that leads to the overestimation of neoplastic cartilage invasion [34]. CT and MR yield very similar results, although neither is ideal for assessing thyroid cartilage invasion in laryngeal cancer. In summary, CT and MR may be considered excellent tests to exclude thyroid cartilage invasion in laryngeal cancer prior to treatment because of their high NPVs and relatively low PPVs.
Metastasis to a paratracheal lymph node (PTLN) in laryngeal carcinoma indicates a worse prognosis. PTLNs, which are nodes along the sides of the trachea, are hard to palpate and evaluate preoperatively using ultrasonography (US). The sensitivity and specificity of CT for the diagnosis of PTLN involvement were 70% and 36%, respectively, whereas those of MRI were 50% and 71%, respectively [35]. However, when radiologic and clinical parameters (subglottic extension and status level I–V) were combined, the sensitivity and NPV were nearly 100%.
A3. What is the role of positron emission tomography (PET)/CT in a preoperative evaluation of laryngeal cancer?
Recommendation 4
PET/CT is recommended for the evaluation of laryngeal cancer, particularly in advanced-stage cases, as it is superior to conventional CT or MR in terms of the accurate detection of regional/distant metastases and second primary cancers (strong recommendation, moderate-quality evidence).
Accurate demarcation of the primary tumor extent and the detection of metastatic disease and second cancers comprise the most important part of pretreatment planning for cancer patients. Currently, contrast-enhanced CT or MRI, US, and fluorodeoxyglucose (FDG)-PET/CT are used to identify the presence and extent of metastatic disease in patients with head and neck cancers, including laryngeal cancer. Several individual studies and metaanalyses have compared the diagnostic accuracies of several different imaging modalities, particularly FDG-PET/CT versus conventional imaging (CT or MR), for metastatic disease detection; however, these studies were not specific for laryngeal cancer.
The National Comprehensive Cancer Network clinical practice guidelines for head and neck cancer recommend performing FDG-PET/CT during the initial staging of patients suspected of having stage III and IV disease of the oral cavity, oropharynx, hypopharynx, and larynx [36]. To date, considerable evidence has demonstrated the superior diagnostic accuracy of FDG-PET/CT in the initial staging of head and neck squamous cell carcinoma (HNSCC) when compared with standard conventional imaging. Further, a recent systematic review indicated the cost-effectiveness of combined FDG-PET/CT scanning of patients with HNSCC; although the expense associated with modality seems high for a screening tool, this procedure reduces the administration of unnecessary additional procedures or treatment offsets [37].
A prospective study of 12 patients with T1–2 staged early glottic cancers demonstrated that 92% of patients had standardized uptake values indicative of malignancy (mean, 4.6; standard deviation [SD], 1.8; 95% confidence interval [CI], 1.2; range, 2.8 to 7.6) and concluded that FDG-PET/CT could be used to identify even early-stage laryngeal cancers [38]. However, given the intrinsically limited spatial resolution of PET/CT imaging and the inability of this modality to adequately assess small-volume lesions, it would be unrealistic to expect that PET/CT would adequately improve the staging of primary laryngeal tumors (T) when compared with endoscopic examination and CT or MR, especially for cases involving early-stage primary tumors [39]. Jeong et al. [40] reported a significantly higher sensitivity for primary laryngeal tumor detection with laryngoscopy than with PET/CT (92.8% vs. 79.4%, P=0.028). These authors also reported the superiority of laryngoscopy plus CT versus PET/CT (P=0.0009 vs. P=0.049) for initial T staging and concluded that PET/CT imaging added no benefit in terms of clinical information when compared with a clinical exam plus CT for the initial evaluation of a patient with glottic cancer [40].
In contrast, a recent meta-analysis (including 24 articles) of the detection of cervical nodal metastases found that the pooled perpatient, per-neck-side, and per-neck-level sensitivities/specificities of FDG-PET/CT were 0.91/0.87, 0.84/0.83, and 0.80/0.96, respectively; these results were higher than those of conventional neck-level imaging (0.63/0.96) [41]. A recent systematic review (including two meta-analyses) also found that FDG-PET/CT could diagnose patients with HNSCC at a high level of accuracy; the authors calculated a pooled sensitivity of 89.3% (95% CI, 83.4% to 93.2%) and specificity of 89.5% (95% CI, 82.9% to 93.7%) for PET/CT and correspondingly, a pooled sensitivity of 71.6% (95% CI, 44.3% to 88.9%) and specificity of 78.0% (95% CI, 30.2% to 96.7%) for standard conventional imaging [42]. Overall, although FDG-PET/CT exhibited good diagnostic performance in the pretreatment evaluation of cervical node metastases in patients with HNSCC, it could not detect disease in half of the patients with metastatic disease and a clinically negative (cN0) neck; among cN0 patients, a sensitivity of 50% (95% CI, 37% to 63%) and specificity of 87% (95% CI, 76% to 93%) were determined [43]. A recent prospective study found that FDG-PET/CT was superior to CT/MRI for depicting occult cervical metastatic nodes in patients with cN0 disease, with perlevel sensitivities of 69% and 39%, respectively (P<0.001) [44].
Regarding the detection of distant metastasis and second cancers, a study including 349 HNSCC patients recommended FDG-PET/CT as a primary staging method, with rates of sensitivity and specificity as high as 97.5% and 92.6%, respectively [45]. A meta-analysis (12 studies between 2001 and 2011) calculated pooled sensitivity, specificity, and Q* index estimates (with 95% CI) for PET/CT of 0.888 (95% CI, 0.827 to 0.928), 0.951 (95% CI, 0.936 to 0.963), and 0.937 (95% CI, 0.844 to 0.964), respectively [46]. However, the ability of FDG-PET/CT to detect malignancy depends on the site and type of malignancy. A retrospective study revealed the limitation of FDG-PET/CT in the early detection of synchronous upper gastrointestinal tract tumors; specifically, the detection sensitivities for synchronous esophageal cancer were as follows: 0% for T1a, 60% for T1b, 0% for T2, 100% for T3, and 100% for T4 [47].
Overall, FDG-PET/CT exhibits good sensitivity and specificity versus MRI or CT alone in the initial staging of laryngeal cancer patients and would be a useful pretreatment diagnostic modality, especially for subjects with advanced-stage tumors. However, in terms of the primary tumor evaluation, an endoscopic examination with CT/MRI or fused PET/MRI is more accurate than FDG-PET/CT, although elucidation of this technique in further studies is required [48].
A4. What is the role of ultrasonography in the staging of laryngeal cancer?
Recommendation 5
For laryngeal cancer staging, US can be used to localize the primary focus and assess the tumor extension, including the cervical nodal status, in a manner complementary to conventional CT/MRI (weak recommendation, low-quality evidence).
To date, the diagnostic role of US has been undervalued in the field of laryngeal cancer because of the fundamental limitation of this modality. That is due to the low penetration of ultrasound because of air in the larynx and calcified cartilage in older male patients. However, recent technological innovations in US, which have yielded increased resolution and real-time image processing, provide concrete soft tissue discrimination around the larynx, regardless of patient movement; accordingly, the usefulness of US, which is non-invasive and therefore advantageous, is being revisited.
Clinical assessments of the laryngeal cancer patients, including the primary tumor site and size, intra-/extralaryngeal spread,and cervical lymph node status, should be documented. Thyroid cartilage, the pre-epiglottic space and paraglottic spaces, and thyroid and other soft tissues that are located in or around the larynx, either anteriorly or superficially, can easily be imaged using US. Such evaluations of the involvement of these adjacent structures are critical because they provide direct proof for tumor staging and treatment plan determination.
A recent retrospective study that compared the diagnostic accuracies of pretreatment US with CT in 72 patients with surgically proven laryngeal cancer reported that the primary tumor detection rate was lower with US than with CT (87.5% vs. 100.0%, P=0.006). Regarding invasion, US and CT yielded similar rates of sensitivity and specificity for most intra- and extralaryngeal structures (P>0.05). On the other hand, US yielded a higher specificity relative to CT in terms of assessments of paraglottic space involvement (94.9% vs. 66.7%, P=0.001). However, an evaluation of vocal cord fixation found no statistical difference between US and laryngoscopy (P=0.223) [29]. In 2001, Tamura et al. [49] reported a pilot study of intralaryngeal US with the filling method during laryngomicroscopic laser surgery. The authors reported that in 10 of 16 cases (63%), it was possible to observe US images in which the mucosal layer structure could be confirmed and concluded that their imaging technique would be particularly useful for determining tumor margins during laser surgery [49]. In summary, laryngeal US can be used as a supplementary imaging tool; however, relatively few studies have validated the usefulness of US for assessments of the primary tumor extent and stage in patients with laryngeal cancer.
One of the most influential prognostic factors affecting patients with head and neck cancer is the presence of metastases to the cervical lymph nodes; accordingly, accurate determination of lymph node involvement is a prerequisite for the development of individualized therapy for patients with laryngeal cancer. According to a review of the literature with regard to single imaging modalities, US yielded superior accuracy when compared with palpation (72.7% vs. 69.7%) for the detection of lymph node metastases of laryngeal cancer, but was inferior to CT (84.9%) and MR (85%). US-guided fine needle aspiration cytology yielded an accuracy of 89%, similar to PET (90.5%) [50]. In contrast, another study of cases with previously clinically undetected metastatic cervical nodes that were identified by US found that US could facilitate laryngeal cancer upstaging by allowing more precise nodal evaluation [51]. Taken together, the above findings suggest that US is valuable in terms of evaluating cervical nodal involvement in patients with laryngeal cancer, although it should be used in combination with other imaging modalities.
Compared with CT, the US detection rate of primary laryngeal cancer was lower in most previous studies. The inability to detect tumors on US scans in patients with early laryngeal cancers was attributed to masking from the almost complete calcification of thyroid cartilage [29]. In another study of glottic cancer, most lesions that were not detected on US images were T1 stage tumors [52]. In contrast, another study of 30 glottic cancers reported a high detection rate (96.7%), although this was likely related to the lower frequency of T1 cancers among the study subjects [53]. These results suggest that the ability of US to detect early glottic carcinomas is limited, especially in patients with highly calcified adjacent cartilages. Evaluations of some laryngeal subsites, such as aryepiglottic folds, the posterior commissure, or posterior lamina of cricoid cartilage, which are usually not clearly visualized by US, would be also limited. Other possible limitations of US include the impossibility of determining reproducibility and the interobserver reliability. Therefore, US has a relatively limited diagnostic value as a single imaging modality for laryngeal cancer staging, and would be better used adjunctively to other imaging tools.
A5. How we can evaluate patients’ preoperative general conditions?
A5-1. Assessment of patients who are eligible for laryngectomy
Recommendation 6
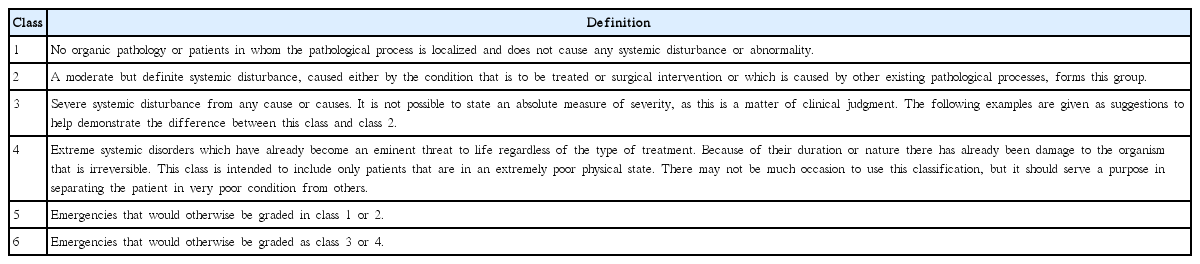
(A) In addition to an anesthesia-related assessment of general health, preoperative pulmonary function test and arterial blood gas levels should be checked in laryngeal cancer patients who have chronic obstructive pulmonary disease (COPD), are older than 60 years, are American Society of Anesthesiologists (ASA) class II or greater, exhibit functional dependence, and have congestive heart failure (strong recommendation, moderate-quality evidence).
(B) If the patient is eligible for partial laryngectomy, a preoperative assessment of pulmonary status and careful review of the patient’s exercise tolerance should be conducted (strong recommendation, low-quality evidence).
The workup required for a laryngectomy includes an anestheticrelated assessment of general health and specific tests relevant to laryngectomy [54-56].
Preoperative posteroanterior and lateral chest radiography of patient may be mandatory when planning laryngeal surgery [56]. In patients with additional risk factors, including COPD, age older than 60 years, ASA class II or greater (Table 5), functional dependence, and congestive heart failure, pulmonary function tests (spirometry and flow-volume loops) could be very useful [57]. The effectiveness of preoperative and surgical countermeasure can be assessed by quantitative measurement of ventilation. Spirometry data is used not only to distinguish restrictive from obstructive pulmonary disease but also to forecast perioperative pulmonary complications. Universally, less than 75% of forced expiratory volume in 1 second (FEV1)/vital capacity (VC) ratio is regarded abnormal, and less than 50% of the ratio indicates significantly increased risk of perioperative pulmonary morbidities [56].
In patients with pulmonary disease, preoperative room air arterial blood gas analysis (ABGA) is recommended. Patients with less than 60 mmHg of arterial oxygen pressure or greater than 50 mmHg of carbon dioxide pressure are tend to develop pulmonary distress after surgery. Consecutive ABGA can also be used to validate the effectiveness of respiratory or medical intervention. In addition, preoperative ABGA as well as chest X-ray give a baseline for postoperative comparison [56].
The preoperative management of underlying pulmonary disease is critical and should consult to a pulmonologist. Smoking have to be prohibited for at least 1 week before surgery. Interventions such as preoperative incentive spirometry or deep-breathing exercises, and the use of intraoperative nasogastric tube decompression can successfully reduce pulmonary complications in high-risk patients. Acute exacerbation of pulmonary disease or infection should be cleared with antibiotics and chest physiotherapy before surgery [58].
If the patient is eligible for partial laryngectomy, a preoperative assessment of the pulmonary status and careful review of the patient’s exercise tolerance are especially important because the patient’s preoperative pulmonary reserve is an important indicator of how well the patient will tolerate postoperative aspiration [57,59].
Clinical parameters such as stair-climbing or block-walking comprise the most important parameter predictive of complications. An incapable to climb two flights of stairs because of dyspnea would contraindicate conservation surgery [60].
Although the FEV1 and VC cannot predict the development of pulmonary morbidities after partial laryngectomy, a FEV1 or FEV1/forced VC below 75% tend to be associated with increased pulmonary morbidities in patients who have undergone partial laryngectomy [60].
Because the presence of COPD does not aggravate the complication rate, the presence of COPD does not contraindicate conservation surgery [58,60].
Nevertheless, no existing objective standards can reliably distinguish which patients can tolerate the physiological changes that accompany conservation laryngeal surgery [57,60].
A5-2. Screening assessment of second primary cancers (synchronous and metachronous head and neck carcinomas)
Recommendation 7
(A) Patients with laryngeal cancer should be examined carefully to detect secondary malignancies (strong recommendation, moderate-quality evidence).
(B) Additional modalities such as chest radiography, CT (chest/abdomen), PET/CT, and panendoscopy are recommended for secondary malignancy screening (strong recommendation, moderate-quality evidence).
Patients with HNSCC often have a history of alcohol and nicotine abuse and therefore have an elevated risk of developing synchronous and/or metachronous squamous cell carcinoma in other parts of the upper aerodigestive system [61]. The reported annual incidence of second primary malignancy (SPM) in HNSCC patients is approximately 3% to 7%, and patients with a previous history of HNSCC have an approximately 14% chance of developing a SPM. Patients with initial HNSCC also have a high rate of secondary cancer, with 41% and 59% developing synchronous and metachronous tumors, respectively. The potential to develop a secondary malignancies within 5 years after undergoing treatment for an initial HNSCC was 22%. Screening and chemoprevention programs should be recommended to the patients with initial HNSCC [62]. Generally, oral cavity and oropharyngeal squamous cell cancers are more frequently associated with head and neck region SPM, whereas laryngeal and hypopharyngeal cancers are more associated with lung SPM [61].
SPMs compromise overall survival of HNSCC patients. The survival of patients with HNSCC and SPM has been shown to be poorer than that of HNSCC patients without SPM (38% vs. 49% at 10 years). The early detection and staging of SPMs has an important impact on treatment and is therefore highly important. A majority of SPMs are detected at an early stage, when curative treatment is still an option. In particular, many patients have curable lung cancers. Taken together, patients with HNSCC, including laryngeal cancer, should undergo careful physical examinations and regular follow-ups to detect SPMs.
Routine workups for metastases of laryngeal carcinoma are essential. The lung is the most frequent site of distant metastasis, followed by the liver, and accordingly chest radiographs and laboratory investigations of liver function, with possible liver US, are the minimal standards at several institutions [63]. Patients with abnormal chest radiography findings and those with advanced disease or a strong clinical suspicion may warrant CT scanning of the chest or abdomen [61].
Recently, FDG-PET/CT is usually included in the initial staging work-up of a patient with laryngeal cancer [57]. FDG-PET/CT is quite better than morphological imaging modalities such as CT or MRI in terms of assessing the primary tumor, lymph nodes, potential distant metastases, and SPMs in a single examination. However, the method by which FDG-PET/CT should be integrated into the staging algorithms of the disease remains controversial [64]. FDG-PET/CT detects many synchronous primaries and seems to be an ideal tool for the guidance of metabolically active lesion biopsies; consequently, panendoscopy can be performed more sufficiently when using information gained from PET [65].
However, the limited spatial resolution of FDG-PET/CT may render small and superficially growing tumors of the aerodigestive system invisible [66]. Therefore, FDG-PET/CT will not substitute routine panendoscopy [65]. The use of panendoscopy for SPM surveillance has been reinforced by several studies. In a study of 200 patients with HNSCC, most metachronous tumors were found to involve the lung and esophagus, and index tumors were diagnosed within 1 to 3 years of therapy [67]. Haughey et al. [68] recommended endoscopic examinations at routine intervals within 2 years of head and neck treatment to ensure the optimal detection of SPMs, as half of all aerodigestive tract SPMs are detected within 2 years of the index tumor diagnosis.
Therefore, concurrent evaluation of FDG-PET/CT and PET/CT-guided endoscopic exam might be the most sensitive strategy for detecting synchronous tumors at early and curable condition. The efficacy of this tactic to improve outcomes with regard to oncologic outcome and cost-effectiveness must be evaluated in the future [61,65].
A5-3. Risk factors for laryngeal cancer
Recommendation 8
A person who reports smoking and drinking habits should undergo regular medical check-ups for laryngeal cancer. Patients who experience voice changes should be sent for a consultation with ENT specialists (strong recommendation, moderate-quality evidence).
Laryngeal cancer is a multifactorial disease associated with a variety of lifestyle factors, environmental factors, and other host factors. Smoking is the predominant risk factor for laryngeal cancer [69]. The combined consumption of alcohol and tobacco increases the laryngeal cancer risk in a synergistic, rather than additive, way. Chronic alcohol consumption affects carcinogenesis through malnutrition and the depletion of vitamins and minerals that protect against cancer [69-73]. Tobacco and alcohol use deteriorate treatment efficacy for laryngeal cancer. The laryngeal cancer patients who maintain smoking and/or drinking are less likely to be cured and apt to develop a secondary malignancies [73]. Current smokers have a 10- to 20-fold increased risk of laryngeal cancer when compared with nonsmokers [74,75]. However, these risks decline after smoking cessation, although never to the same level as that of patients who have never smoked. There is an approximately 60% reduction in the relative risk at 10 to 15 years after smoking cessation [76].
Alcohol consumption can increase the risks of cancer of the mouth, throat, esophagus, larynx, liver, and breast. People who take 50 or more grams of alcohol per day (approximately 3.5 or more drinks per day) have at least a 2- to 3-fold greater risk of developing such cancers, compared with nondrinkers [77]. The risk of cancer is much higher for individuals who take both alcohol and tobacco. Moreover, the risks of these cancers are significantly higher among people who consume such high amounts of alcohol while using tobacco [78].
A lower socioeconomic status, which results in poor health care, smoking, drinking, and dietary habits, and exposure to environmental and occupational carcinogenic factors have been associated with cancer. All of these factors are possible explanations for the increased risk of laryngeal cancer [69-80].
Other risk factors include carcinogens in the workplace, such as asbestos, nickel compounds, wood dust, leather products, paint, diesel fume, and glass-wool [81]. A potential association with chronic gastroesophageal reflux disease or laryngopharyngeal reflux disease remains controversial [82,83].
Furthermore, the relationships between the increased incidence of SCC, laryngeal papillomatosis and human papilloma virus (HPV) remain controversial. Although good evidence supports a causal link between HPV subtypes 16 and 18 and oropharyngeal cancer, the association with laryngeal cancer is uncertain [84,85].
B. Premalignant laryngeal lesions
B1. What is the appropriate management for a premalignant laryngeal lesion?
B1-1. Definition of a premalignant laryngeal lesion
The World Health Organization classifies premalignant laryngeal lesions as either hyperplasia; keratosis; mild, moderate, or severe dysplasia; or carcinoma in situ [86,87]. Very early lesions may exhibit hyperkeratosis or parakeratosis without cellular atypia or dysplasia. Squamous cell dysplasia is characterized by cellular atypia and a loss of normal maturation and stratification. Cellular abnormalities associated with mild dysplasia are limited to the basal third of the epithelium; whereas, moderate dysplasia shows marked cellular abnormalities involving up to two-thirds of the epithelium, and severe dysplasia is characterized by cellular abnormalities involving more than two-thirds of the epithelium. Carcinoma in situ is an intraepithelial neoplasm in which the full thickness of the squamous epithelium exhibits the cellular features of carcinoma without violation of the basement membrane.
B1-2. Diagnostic procedure for a premalignant laryngeal lesion
Recommendation 9
Although various endoscopic and imaging techniques could help physicians to predict whether a lesion is malignant or benign, biopsy is the gold standard for diagnosis (strong recommendation, moderate-quality evidence).
The visual appearance of a premalignant laryngeal lesion does not predict its histologic nature, nor does laryngeal videostroboscopy reliably differentiate premalignant from malignant lesions [88]. The use of vital dyes, including toluidine blue and methylene blue, has been explored [89,90]. Toluidine blue yielded a 91% sensitivity but only 52% specificity for the detection of dysplasia or malignant changes [90]. Contact endoscopy with methylene blue staining provides a magnified image with histologic information and an assessment of vascular patterns [91]. However, this technique is inadequate for characterizing thicker lesions. Regarding autofluorescence endoscopy, human tissues contain many compounds that fluoresce when exposed to blue light. The differing fluorescence of abnormal tissues has been exploited as a diagnostic aid for laryngeal malignancy. However, this technique is limited by the possibility of false-positive and false-negative examinations in cases involving scarring, hyperkeratotic lesions, and inflammation [92]. Optical and microscopic imaging is limited by an inability to evaluate the submucosal architecture below the first few layers of epithelial cells. In contrast, infrared light has increased tissue penetrance and can provide diagnostic information about subsurface tissues. Optical coherence tomography uses near-infrared light waves to examine the epithelial and subepithelial architecture waves [93]. Therefore, optical coherence tomography is a potentially useful tool in the management of laryngeal cancer.
NBI is a new technology that uses blue and green light (respective wavelengths: 415 and 540 nm) to observe the microvascular structure in the epithelium. Superficial mucosal lesions that cannot observed with white light endoscopy could be identified by their angiogenic patterns on NBI. Ni et al. [94] devised a five-type classification system of laryngeal leukoplakias that incorporated the vascular pattern of the intrapapillary capillary loop, and reported a correlation between their classification system and pathologic findings. Subsequently, Bertino et al. [14] analyzed premalignant laryngeal lesions using the Ni classification. In that study, NBI yielded a sensitivity, specificity, accuracy, PPV, and NPV of 97.4%, 84.6%, 92.7%, 91.6%, and 95.1%, respectively [14]. Compared with autofluorescence, NBI showed superior specificity for the detection of early neoplastic lesions [92,95-97]. However, endoscopic analyses should always be confirmed by histopathologic lesion analyses.
B1-3. Approach for a premalignant laryngeal lesion
Recommendation 10
(A) Either an intervention or follow-up protocol can be recommended for cases of mild and moderate dysplasia (weak recommendation, moderate-quality evidence).
(B) Intervention is recommended for cases of severe dysplasia/carcinoma in situ (weak recommendation, moderatequality evidence).
As the lesions of moderate dysplasia progressed to invasive cancer in 0% to 45%, medical or surgical intervention was recommended in these cases [80,98-105]. Dysplastic lesions could be excised using microlaryngoscopic techniques to remove the visible lesion. Close follow-up is required because of the risk of recurrence of the lesion and possible malignant transformation. In patients with lesions of mild dysplasia, it progressed to invasive cancer in 0% to 11.5%. Therefore, a regular follow-up is usually recommended.
Severe dysplasia and carcinoma in situ have the similar high risk of progression to invasive carcinoma and they are considered as the same disease entity for clinical purposes [98]. Standard treatment strategies have not established in laryngeal lesions of severe dysplasia or carcinoma in situ yet. Practices implemented in different environments are likely based on consensus rather than on a high level of evidence from the literature [86,106]. In previous studies, watchful waiting policy has failed to manage the lesions of severe dysplasia/carcinoma in situ, because the lesions progressed to invasive cancer in most cases [99,100]. Therefore, medical or surgical treatment should be performed in all cases of severe dysplasia/carcinoma in situ. The treatment method of these lesions includes radiotherapy, CO2 laser excision, vocal cord stripping, and so on [98]. Radiation therapy is generally not recommended for the treatment of premalignant lesions of the larynx. However, this modality is recommended on rare occasions for high grade dysplastic lesions with poor access [107].
B1-4. Follow-up of premalignant lesions
Recommendation 11
All patients with varying grades of dysplasia upon pathologic examination should be followed up (strong recommendation, low-quality evidence).
Patients with severe dysplasia and carcinoma in situ should be kept under surveillance in a manner similar to that for early laryngeal carcinoma: every 1 to 3 months for the first year, every 2 to 6 months for the second year, every 3 to 6 months during the third year, and every 6 months during years 4 and 5. Patients with mild or moderate dysplasia and risk factors (continued smoking, persistent hoarseness, and visible lesions) should also be observed for at least 6 months. Patients who have mild or moderate dysplasia without risk factors are considered as low-risk group. Opinions vary widely with regard to the duration of follow-up for these patients. Some clinicians recommend at least a 2-year follow-up. Others recommend early discharge from the clinic and an early return if symptoms develop [107].
C. Glottic cancer
C1. What is the appropriate surgery for a primary T1/T2 glottic cancer?
Recommendation 12
(A) Transoral laser microsurgery is recommended for the achievement of acceptable oncologic and functional outcomes in patients with T1/T2 glottic cancer (strong recommendation, moderate-quality evidence).
(B) Transoral laser microsurgery can be recommended as a treatment option for T1/T2 glottic cancer with anterior commissure involvement if adequate resection margin can be obtained (weak recommendation, moderated-quality evidence).
(C) Open partial laryngectomy may be a good surgical option for the achievement of acceptable oncologic outcomes and functional preservation in cases of T1/T2 glottic cancer with limited extension into adjacent subsites or the anterior commissure (weak recommendation, moderate-quality evidence).
Transoral laser microsurgery is gaining popularity for the management of early glottic cancers, as it has been associated with voice preservation, a shorter treatment duration, and similar survival rates as radiotherapy according to a case series [108-114]. In addition, transoral laser microsurgery has some benefit compared to the conventional open partial laryngectomy, such as low morbidity, a reduced necessity of tracheostomy and/or nasogastric feeding, short hospital stay, and few sequelae related to surgical procedures [115]. Transoral laser microsurgery can be easily repeated and affords more available retreatment options for local recurrence, compared to initial radiation therapy or open partial laryngeal surgery [116]. Furthermore, transoral laser microsurgery is the lowest-price treatment modality, followed by radiation therapy [117].
Several recent reports have confirmed the efficacy of transoral laser microsurgery for the treatment of early glottic cancer. The reported local control rates of transoral laser microsurgery in patients with T1a and T1b glottic cancer range from 86% to 93%, with a laryngeal preservation rate of approximately 95% [118-120]. In 2007, Hartl et al. [118] reported the treatment outcomes of 142 patients with Tis, T1a, and T1b disease who were underwent surgical procedures with curative intent using five types of cordectomy, determined by existence of tumor involvement. The overall 5-year recurrence-free survival rate was 89%, and the 5-year disease-specific survival rate was 97.3%. In 2008, Sjogren et al. [120] reported local control and larynx preservation rates of 89% and 96%, respectively, among 189 patients with T1a glottic cancer who had been treated since 1996. In addition, the estimated 5-year overall survival rate in patients with T2 glottic cancer was reported to be as high as 93% after transoral laser microsurgery [119].
Currently, transoral laser microsurgery is generally used particularly in patients with T1–T2 glottic cancer [121]. Regarding the increase of clinical experience with transoral microsurgery, the application of transoral laser microsurgery will be further extended to include more extensive laryngeal cancers, even though radiation therapy may promise better vocal outcomes when patients need extensive cordectomy [116]. In addition, a small subset of transoral laser surgeons have successfully used this technique to treat moderately advanced cancers [110]. For extension of transoral laser microsurgery, close cooperation with expert pathologists is required.
In conclusion, transoral laser microsurgery can provide excellent oncologic outcomes for early glottic cancer, provided that adequate surgical fields and the surgeon’s experience are guaranteed [113,118-124]. Low morbidity and mortality and less hospital stays make transoral laser microsurgery an attractive therapeutic alternative to conventional open partial laryngectomy.
Conservation open laryngeal surgery encompasses a broad array of open surgical techniques ranging from a laryngofissure approach with cordectomy to SCL. Rarely, the laryngofissure approach with cordectomy might be required for patients with poor transoral exposure [125]. However, the emergence of many literatures providing the oncological and functional benefits of transoral laser microsurgery have resulted in even fewer indications for open partial laryngectomy [113,126,127].
Vertical partial laryngectomy, also known as hemilaryngectomy, is a time-honored approach to resection of an entire ipsilateral glottic larynx, including the paraglottic space and corresponding thyroid ala, while preserving the ipsilateral arytenoid. The local control rates for T1 cancers range from 89% to 100% [128-130]. Involvement of the anterior commissure decreases local control; several studies showed that anterior commissure involvement decreased the local control rate from 93% to 75% [128-131]. An extended vertical partial laryngectomy or frontolateral vertical hemilaryngectomy could be performed for tumors involving the anterior fold. However, vertical partial laryngectomy may be less effective in patients with large T2 disease.
SCL is an organ-preserving surgical technique for early-stage glottic cancer. The 5-year local control rate associated with supracricoid partial laryngectomy among patients with early glottic cancer with anterior commissure involvement was as high as 98.2%. The approximate overall survival rate ranges from 86% to 93% [132-134]. In addition, several studies have reported local control rates exceeding 80% following open partial laryngectomy, even in patients with T3 and T4 disease [135-138]. Of note, Eckel [109] insisted that supracricoid partial laryngectomy should be considered as a treatment option for tumors involving the anterior commissure or unfavorable T2 tumors, as this technique yields superior local control when compared to transoral laser microsurgery.
A recent systematic review of the oncologic outcomes of open partial laryngectomy for all stages of laryngeal cancer demonstrated that excellent oncologic outcomes could be achieved with this modality; at 24 months, the estimated local control rate was 89.8%, the estimated overall survival rate was 79.7%, and the pooled mean disease-free survival rate was 84.8% [139]. However, the role of open partial laryngectomy for early glottic cancer management has been reduced during the past decade, as many surgeons prefer transoral laser microsurgery for early-stage cases.
In conclusion, open partial laryngectomy should be considered for selected tumors when the outcomes of radiation are less optional and transoral laser microsurgery is not feasible because of local extension to an adjacent site, tumor bulk, or difficulties with access [140]. In addition, specific expertise is needed to ensure reproducible results from open partial laryngectomy, as this technique is associated with several special challenges in terms of patient selection, surgical technique, and postoperative care.
The treatment of early glottic cancer involving anterior commissure is controversial because such involvement may be related with increment of local recurrence rate. The anatomy and impact of the anterior commissure were the subjects of several investigations and remain controversial [141-145]. The some authors regard the anterior commissure as a weak point to tumor invasion [141,146]. They suggest that the anterior commissure is a route of invasion into the thyroid cartilage, because there are no existence of perichondrium/periosteum at the insertion of Broyles’ ligament. Whereas others consider that the anterior commissure tendon might be a barrier to prevent invasion into the thyroid cartilage [142,143].
The therapeutic options of early glottic cancer involving anterior commissure still remain as a controversy in spite of several advantage of transoral laser microsurgery. Some cases with anterior commissure involvement, there are the increased difficulty of tumor exposure and these would be led to the requirement for significant surgical experience. For these situations, open partial laryngectomy techniques including frontolateral partial laryngectomy or supracricoid partial laryngectomy are considered as other possible treatment options with comparable oncologic outcomes; however, these are associated with a greater risk of surgical morbidities, such as voice quality and decannulation issues and aspiration [147,148].
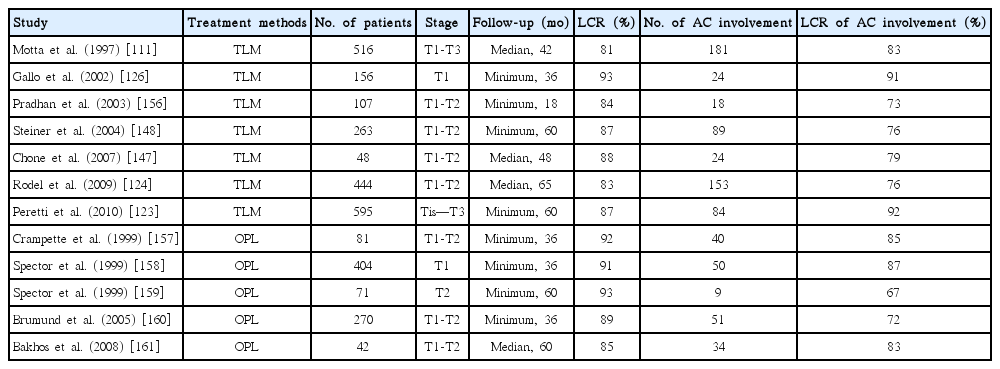
Some authors believe that the anterior commissure involvement would be a contraindication to perform transoral laser microsurgery because of higher local recurrence rate [149-151]. In contrast, others have indicated that early glottic cancers invading to the anterior commissure can be successfully treated with transoral laser microsurgery [152,153]. Pearson and Salassa [154] reported outstanding results when employing transoral laser microsurgery for glottic cancer with involvement of the anterior commissure. Motta et al. [111] reported a study of 516 patients with glottic cancer (T1–T3). Their series enrolled 127 patients with T1b stage tumor, for which a 5-year disease-free survival of 83% was accomplished. Peretti et al. [155] observed a slight decrease (83% vs. 87%) in local control among patients with anterior commissure involvement. As described in Table 6, when compared with open partial laryngectomy, transoral laser microsurgery could provide an acceptable local control rate (73% to 91%) for early glottic cancers with anterior commissure involvement [111,123,124,126,147,148,156-161].
In conclusion, transoral laser microsurgery might be sufficient for the treatment of early glottic cancer with anterior commissure involvement, assuming the guarantees of adequate surgical field exposure and surgeon experience. Otherwise, surgical alternatives such as open partial laryngectomy should be considered for the achievement of acceptable oncologic outcomes, as anterior commissure involvement is a major risk factor for decreased local control [148].
C2. What is the proper surgical management for T3/T4 glottic cancer?
Recommendation 13
(A) Total laryngectomy should be considered as the primary surgical modality for T3/T4 glottic cancers (strong recommendation, low-quality evidence).
(B) In selected T3/T4 glottic cancers, open partial laryngectomy can be performed to maintain laryngeal function, although the increased postoperative morbidity with this procedure, compared to total laryngectomy, should be considered (weak recommendation, low-quality evidence).
(C) Surgical management of the thyroid gland in cases involving a subglottic extension exceeding 10 mm, transglottic tumors, and a subglottic subsite should include at least ipsilateral lobectomy and isthmectomy (strong recommendation, high-quality evidence).
Canis et al. [162] observed a 5-year local control rate of 71.5% and larynx preservation rate of 83% when using transoral laser microsurgery for the treatment of pT3 glottic laryngeal carcinoma. The 5-year overall survival, recurrence-free survival, and disease-specific survival rates with this procedure were 58.6%, 57.8%, and 84.1%, respectively [162]. In cases with large tumors, visualization of both the deep and surrounding structures is impaired, and the tumor is removed piecemeal during transoral laser microsurgery; accordingly, the potential for a positive margin increases, and postoperative radiotherapy is required. The results achieved with transoral laser microsurgery are similar to those after conventional total laryngectomy and showed better results than those achieved with primary chemotherapy or radiotherapy. Therefore, transoral laser microsurgery, regardless of adjuvant radiotherapy, is effective treatment for organ preservation. Furthermore, transoral laser microsurgery with low morbidity and mortality and excellent oncologic and functional outcomes can be an attractive therapeutic option for T4a laryngeal cancer [163]. For T3 glottic carcinoma, total laryngectomy is often performed with neck dissection, with or without adjuvant radiotherapy. Locoregional control and 5-year overall survival rates were reported from 69% to 87% and from 53% to 56%, respectively [164-166]. The panels have suggested the recommendation about transoral laser surgery in selected T3/T4 cases with weak recommendation and low-quality evidence. However the recommendation was removed from manuscript because it failed to get agree of more than 2/3 of Delphi panels even in the second round when the indication was limited to only T3.
In selected T3/T4 cases, hemilaryngectomy can be an alternative surgical option to total laryngectomy. In hemilaryngectomy cases, local control rates and 5-year overall survival rates range from 73% to 83% and from 71% to 75%, respectively [162, 167-169]. Another surgical option for the treatment of selected T3 glottic cancers is supracricoid laryngectomy with cricohyoidoepiglottopexy (SCL-CHEP). Chevalier et al. [170] and Piquet and Chevalier [171] analyzed 112 glottic carcinoma patients with impaired vocal fold mobility (n=90) or fixation (n=22). The authors reported a local control rate of 97.3% and 5-year larynx preservation rate of 95.5%. The contraindications for SCL-CHEP are fixation of the arytenoid cartilage, tumor spread to the upper border of the cricoid cartilage, infiltration of the cricoid or thyroid cartilage, extensive infiltration of the pre-epiglottic space, and extralaryngeal spread [172]. In contrast, these limitations are not contraindications for transoral laser microsurgery. Moderate oncological results have been reported for transoral laser microsurgery, either with or without neck dissection and adjuvant (chemo) radiotherapy. Vilaseca and Bernal-Sprekelsen [173] analyzed 167 patients with pT3 glottic carcinoma who were treated with transoral laser microsurgery. The 5-year local control rate, the secondary laryngectomy and the 5-year recurrence-free survival rate was 68%, 14.3%, and 62%, respectively.
SCL shows a high level of functional outcomes and therefore can replace near-total laryngectomy as an organ-sparing surgical option. Local control rates of SCL is greater than 96%, as well as improved swallowing and speech quality-of-life measures compared to total laryngectomy [174,175]. Benito et al. [176] investigated the swallowing outcomes of a series of 457 patients who underwent SCL, including normal swallowing without aspiration in 259 (58.9%), subclinical grade 1 aspiration in 87 patients (19%), and severe grade 2 or 3 aspiration in 101 patients (22.1%). In this study, greatest risk factors for severe aspiration were older than 70 years and had undergone cricohyoidopexy (CHP) with partial or total arytenoid resection. Aspiration could be managed via temporary gastrostomy, permanent gastrostomy, and completion total laryngectomy in 34.5%, 1.6%, and 3.7% of the patients, respectively. In T2 and select T3 cases, the local control rates were greater than 90% and are therefore similar to the rates achieved with chemoradiotherapy or total laryngectomy [169,177]. Functionally, despite preservation of speech and swallowing, the postoperative voice quality differs. For swallowing recovery, intensive rehabilitation over several weeks may be required. Despite these obstacles, 80% to 90% of patients will recover their swallowing function within the first year [178]. A T3 tumor with vocal cord fixation is a candidate for SCL if the arytenoid is mobile during endoscopic examination. A fixed arytenoid indicating tumor invasion of the cricoarytenoid joint is not a candidate for SCL. Generally, this procedure would be relatively contraindicated for a pT4 patient who was clinically staged as T3 but exhibited tumor extension through the thyroid cartilage at the final pathologic analysis. Invasion through the outer perichondrium of the thyroid cartilage worsen survival than thyroid cartilage invasion alone [135,179]. Although some researchers have reported the successful treatment of T4 tumors via SCL in which the strap muscles were not dissected from the larynx, this technique is not recommended for the novice [180].
The indications for primary total laryngectomy for advanced laryngeal cancer remain controversial. RTOG 91-11 included patients with minimal cartilage erosion or tongue base involvement. Patients with advanced laryngeal cancer who present with a poor functional status, manifested by severe airway compromise requiring tracheostomy or enteric feeding, are poor candidates for laryngeal preservation [181].
A literature search identified 16 studies involving a total of 1,180 cases that were suitable for inclusion. A systematic review and meta-analysis of all published data and review of case series at Newcastle upon Tyne Hospitals reported that the overall pooled incidence of thyroid gland invasion in these 1,287 patients was 10.7% (95% CI, 7.6% to 14.2%). Patients with primary subglottic tumors (relative risk, 7.5; 95% CI, 4.3 to 13.0) and disease extension into the subglottis (relative risk, 4.3; 95% CI, 2.5 to 7.2) were significantly higher relative risk factors of thyroid gland invasion [182]. Furthermore, the analyses of 399 total laryngectomy specimens, including 33 cases of thyroid gland invasion (8%), were reported. The subsites for these thyroid gland invasion positive cases included glottic, transglottic, subglottic, and supraglottic locations in seven, eight, five, and three cases, respectively; there was no report of subsite in 10 cases. In 17 cases (94%), thyroid gland invasion was by direct extension, whereas invasion by lymphatic spread was observed in only one specimen; the method of thyroid gland invasion was not recorded for 15 specimens. Twenty-three thyroid gland invasion specimens reported subglottic extension, and in all 23, this extension exceeded 10 mm. A subglottic extension greater than 10 mm (P=0.002), transglottic tumor (P=0.025), and subglottic subsite (P=0.018) were all significant risk factors of thyroid gland invasion. Two studies reported and analyzed cartilage invasion. The adjusted pooled odds ratio for the association between thyroid gland invasion and a subglottic extension greater than 10 mm was 10.47 (P=0.0004) [183].
C3. What is the appropriate management of the neck lymph nodes in glottic cancer?
C3-1. Management for clinically positive neck (N+) in patients with glottic cancer
Recommendation 14
(A) Therapeutic neck dissection in patients with N+ glottic cancer should include at least the ipsilateral neck levels II, III, and IV (strong recommendation, low-quality evidence).
(B) Elective contralateral neck dissection is not routinely recommended for ipsilateral N+ glottic cancer (weak-recommendation, low-quality evidence).
Treatment of neck lymph node metastases should be performed according to the presence of clinically positive neck nodes [184]. Radical or modified radical neck dissection could be considered according to the lymph nodes metastasis status. Few studies have reported recommendations regarding the levels of neck dissection in clinically neck-positive glottic cancer, as the specifics of this procedure are normally determined according to disease involvement. Ipsilateral levels II, III, and IV are most frequently involved in the cervical metastasis of advanced glottic cancers [185]. Levels I (1.8% to 5%) and V (2% to 11%) are rarely involved [186-188]. However, the involvement of levels I and V is usually associated with metastases in levels II, III, or IV. Moreover, the involvement of level V increases along with the involvement of other levels in aerodigestive tract SCCs (0% to 15.8% for single-level involvement; 3.2% for two-level involvement; 15.3% for three-level involvement; 40.0% for four-level involvement) [189]. Therefore, dissection of neck level I or V may be considered according to the individual nodal status.
The clinical efficacy and safety of super-selective neck dissection have not yet been evaluated in the context of clinically N+ glottic cancer.
A few studies have evaluated contralateral neck dissection for glottic cancer. In advanced glottic cancer, most metastatic lymph nodes are located at ipsilateral levels II, II, and IV (87.5% to 95%) [185,190,191]. Among lateral glottic cancers, the rate of contralateral neck metastasis was very low (3.5%) [191]. Glottic cancers, including transglottic cases, are associated with a low prevalence of contralateral metastases, even if the primary tumor extends beyond the midline (4%) [192]. Therefore, contralateral neck dissection may not be considered [191,193].
C3-2. Management for clinically negative neck (N0) in patients with glottic cancer
Recommendation 15
(A) Elective neck dissection is not routinely recommended for T1N0 and T2N0 glottic cancers, but should be considered for T3N0 and T4N0 glottic cancers (strong recommendation, low-quality evidence).
(B) In cases of T3N0 and T4N0 glottic cancer, elective neck dissection should include ipsilateral neck levels II, III, and IV (strong recommendation, low-quality evidence).
Generally, elective treatment of the neck is justified if the risk of occult lymph node metastasis exceeds 15% [151]. In previous studies, the rates of occult lymph node metastasis in early glottic cancer (T1–T2) ranged from 0% to 8.6%, and nodal recurrence rarely occurred during follow-up [194-196]. Therefore, elective neck dissection is not recommended for early glottic cancer [110,197]. Among advanced N0 glottic cancers, the neck recurrence rate ranges from 14.3% to 23.4% [185,194,196,198]. Therefore, elective neck dissection is acceptable for advanced glottic cancers without clinical neck metastasis (T3N0 and T4N0) [166,199-203]. However, some studies reported that among patients with T3N0 glottic cancer, follow-up observation involving meticulous examinations and appropriate treatment for subsequent neck disease resulted in a similar survival rate as that of initial neck treatment (treated group 72%, observation group 70%) [166]. Other authors reported the survival rates of patients with T4N0 glottic cancer who underwent initial neck treatment versus those who remained under observation, with later treatment if necessary (5-year disease-specific survival, 31% vs. 44%) [204].
Ipsilateral selective neck dissection of levels II, III, and IV is sufficient for clinically node negative glottic cancer [200,201, 205,206], as the lymphatic spread of glottic cancer to the neck follows a predictable path along the jugular chain [207-209], and levels I and V are rarely involved in a clinically negative neck (level I, 0% to 14%; level V, 0% to 7%) [201,209,210]. This finding was proven by many studies, including well-controlled randomized prospective studies [196,211-213]. Contralateral neck dissection is not recommended for T3N0 and T4N0 glottic cancers, which have a very low contralateral neck metastasis rate [185].
Recently, super-selective neck dissection of levels IIa and III was suggested [184,214]. Sublevel IIb (0% to 9.5%) and level IV (3.4%) are rarely involved in clinically N0 glottic cancers [215-218]. Level IIb sparing could reduce morbidities such as spinal accessory nerve paralysis and injuries to the digastric and sternocladomastoid muscles [218]. Level IV sparing could reduce potential complications such as chylous fistula and phrenic nerve injury [219].
D. Supraglottic cancer
D1. What is the appropriate surgical treatment for a supraglottic primary site?
D1-1. Surgical treatment for T1/T2 supraglottic cancer
Recommendation 16
(A) Conservative laryngeal surgery (open partial laryngectomy or laser/robotic transoral laryngeal surgery) is recommended primarily for the patients with T1/T2 supraglottic cancer (strong recommendation, moderate-quality evidence).
(B) If surgical exposure is inadequate during transoral laryngeal surgery for supraglottic cancer, conversion to another treatment option, such as radiation therapy or open partial laryngectomy, should be considered (strong recommendation, low-quality evidence).
For patients with early supraglottic cancers (T1 and T2 tumors), successful disease control can be achieved by either traditional conservation surgical procedures, including open partial laryngectomy, or curative doses of irradiation [220,221]. Therefore, treatment modality can be decided according to the expected posttreatment functional outcome, the patient’s wishes and general medical condition, and reliability of follow-up. Open partial laryngectomy yields excellent local control of early supraglottic cancers, with reported rates ranging from 80% to 100% [222-225]. Despite the high rates of local tumor control with open partial laryngectomy, the possibility of lung complications by significant aspiration and postoperative dysphagia may frequently preclude the application of this procedure. Because open partial laryngectomy disrupts the pharyngeal muscles, strap muscles, and sensory innervation of the pharynx and larynx, swallowing is markedly impaired, especially in the early postoperative period. Moreover, the adjunctive use of a tracheostomy and feeding tubes is necessary during the early and intermediate postoperative period after open partial laryngectomy due to airway obstruction by laryngeal swelling [226].
With the wide acceptance of transoral laryngeal surgery, extrapolations of less invasive approaches to the supraglottic larynx have been described [227,228]. The oncologic results of transoral laryngeal surgery can be comparable to those of open partial laryngectomy if complete resection is achieved. Moreover, although open partial laryngectomy and radiotherapy yielded comparable functional outcomes [229], transoral laryngeal surgery is generally associated with a lower risk and shorter duration of postoperative morbidity [230-232]. The functional outcomes of transoral laryngeal surgery are superior to those achieved with a conventional open approach with regard to the duration of applying feeding tube and tracheostomy, incidence of pharyngocutaneous fistulae, and lengths of hospital stay [116].
In 1998, Ambrosch et al. [233] reported the outcomes of early (T1 and T2) supraglottic cancer who underwent transoral laryngeal surgery between 1979 and 1991. A 100% 5-year local control rate was achieved for pT1 cases, whereas a rate of 89% was achieved for pT2 cases. The 3-year recurrence-free was 83% and overall survival rates was 76%, respectively. This study revealed that local control and survival of transoral laryngeal surgery were comparable to those of conventional open partial laryngectomy in early supraglottic cancer. Other reports of transoral laryngeal surgery for supraglottic cancer also concluded that transoral laryngeal surgery is an excellent treatment for early supraglottic cancers including selected T3 lesions, if clear margins could be achieved [234,235]. In 2004, Davis et al. [227] reported the outcomes of T2 and T3 supraglottic cancers by laser resection, in which local control was achieved in 97% of patients receiving combined treatment with radiotherapy and in 100% among patients treated with surgery alone. In 2008, Cabanillas et al. [236] retrospectively compared the patients with supraglottic cancer treated by laser surgery and conventional open approaches. The laryngeal preservation rates were 86% and 80% in the laser group and transcervical group, respectively (P=0.6). The larynx was preserved in all patients classified as T1 and T2 who survived for 5 years after surgery. In 2006, Peretti et al. [230] performed study comparing the functional outcomes of transoral laryngeal surgery with conventional open approach. Significant differences were observed with respect to swallowing function (P=0.03), duration of hospital stay (P=0.0001) and feeding tube and tracheostomy duration (P=0.0001). The authors concluded that transoral laryngeal surgery had a significantly lower functional impact on swallowing, compared with the conventional open approaches, and was also associated with reduced postoperative morbidity and a shorter hospitalization duration. Recently, transoral laryngeal surgery via a robotic system was introduced. Several papers reported the comparable surgical and function outcomes of this procedure with those of conventional transoral laser surgery; however, a longer follow-up duration and larger observational cohort may be needed to establish the role of transoral laryngeal surgery in supraglottic cancer treatment [237,238].
One important consideration of transoral laryngeal surgery is that adequate exposure is necessary to ensure a proper resection [235,239]. Even with experienced hands, complete resection was impossible in approximately 8% to 10% of cases [235]. In addition, approximately 40% of patients in whom R1 and R2 resection were achieved with transoral laryngeal surgery failed to reach complete remission after initial treatment modalities and finally died by tumor progression. Therefore, if the surgical extent is inadequate, other treatment option including radiation therapy or open partial laryngectomy should be considered for proper tumor resection. A skilled surgical technique and experience are important factors in a successful resection, and the possibility of conversion to open partial laryngectomy or a change to postoperative radiotherapy should be addressed with the patient before surgery. In summary, early supraglottic cancer (T1/T2) can be managed via transoral laryngeal surgery (with adequate surgical exposure) with or without postoperative radiation therapy, and favorable local control and survival outcomes can be achieved. A majority of studies that compared transoral laryngeal surgery with open surgery for supraglottic cancer (mostly case series or case-control studies) demonstrated comparable oncological outcomes with superior functional results, especially with regard to swallowing.
D1-2. Surgical treatment for T3/T4 supraglottic cancer
Recommendation 17
(A) Total laryngectomy can be considered as the primary surgical treatment for T3/T4 supraglottic cancer (strong recommendation, low-quality evidence).
(B) Partial laryngectomy can be performed to maintain laryngeal function in selected T3/T4 supraglottic cancers without extensive tongue base invasion, bilateral cricoarytenoid unit impairment, or inferior extension to the cricoid cartilage, although the risk of increased postoperative morbidity relative to total laryngectomy should be considered (weak recommendation, low-quality evidence).
Traditionally, survival has been used as the endpoint when assessing the best treatment option; however, consideration of several parameters including posttreatment functional status, organ preservation, treatment costs, and quality of life have been increasingly emphasized during the last two decades [240]. Until 1950, total laryngectomy and radical neck dissection and followed by radiotherapy was the only procedure accepted for the treatment of laryngeal cancer, and was associated with cure of disease by approximately 60% to 70% [240-242]. Today, however, the goal of treatment focuses not only on a cancer cure, but also on the preservation of laryngeal function [243].
Although the surgical excision of advanced primary tumors has traditionally been achieved via total laryngectomy, which remains the most commonly used procedure, conservative laryngeal resection may be used in selected cases. In 2007, a prospective study evaluated patients who underwent transoral laryngeal surgery for supraglottic cancer [244]. In addition to T1/T2 cases, this study included selected T3 and T4 cases (eight patients, 21%). The 2-year local control, loco-regional control, disease-specific survival, and overall survival were 97%, 94%, 80%, and 85%, respectively. In addition, 79% of the patients finally had overall functional laryngeal preservation. In addition, Canis et al. [245] also reported excellent oncologic outcomes with better functional outcomes of transoral laryngeal surgery for supraglottic cancer. These authors evaluated 277 patients and concluded that transoral laryngeal surgery yields a low rate of morbidity, fast recovery, and superior postoperative function when compared to standard therapy [245].
As described for early supraglottic cancer treatment, advanced supraglottic cancer (T3/T4) can be managed with supraglottic laryngectomy or SCL [246]. T3 tumors with pre-epiglottic space invasion but without transglottic spread may be good candidates for supraglottic partial laryngectomy (SPL). Cases requiring arytenoidectomy will also require extended SPL. However, this procedure is often associated with a longer recuperative duration and increased incidence of serious complications [247]. In addition, SCL may be another option for lesions with glottic extension. The oncologic results of SCL are excellent, with 5-year survival rates and local control rates of 67% to 95% and 88% to 95%, respectively [137]. SCL can be used for the following selected T2, T3, and T4 supraglottic and transglottic tumors: T2 tumors involving the true vocal cords or anterior commissure, extension to the floor of the ventricle, and/or impaired motion of the true vocal cord; T3 transglottic and supraglottic tumors with true vocal cord fixation and/or pre-epiglottic space invasion; and T4 transglottic and supraglottic tumors with limited invasion of the thyroid cartilage but without extension to the outer thyroid perichondrium, or extralaryngeal spread [248-250]. In summary, for T3 or T4a supraglottic tumors without extensive tongue base involvement or cartilage destruction, conservative laryngeal surgery may be used primarily for functional larynx preservation. For tumors with extensive tongue base invasion, bilateral cricoarytenoid unit impairment, or inferior extension to the cricoid cartilage, total laryngectomy remains the preferred initial treatment.
D2. What comprises appropriate neck lymph node management in supraglottic cancer?
D2-1. Management for clinically positive neck (N+) in patients with supraglottic cancer
Recommendation 18
Therapeutic neck dissection should be performed for N+ in patients with supraglottic cancer. The extent of neck dissection should include at least level II, III, and IV (strong recommendation, low-quality evidence).
Neck management of supraglottic cancer requires a different philosophy than that of glottic cancer because in the former, the lymphatic system is involved at a much earlier disease phase and neck nodal metastases are much more common [251]. Appropriate cervical lymph node treatment is an important aspect of therapy for patients with supraglottic cancer, as the nodal status has prognostic significance [252-255]. The presence of clinically palpable cervical lymph node metastasis is associated with an approximately 50% reduction in overall survival [252-256]. The cervical metastasis sites are well-defined, and the most common nodal metastasis sites are cervical levels II, III, and IV. Candela et al. [188] retrospectively reviewed 247 consecutive patients with supraglottic cancer who underwent comprehensive neck dissection. An analysis of the lymph node metastasis distribution revealed a remarkable preference for levels II (62%), III (53%), and IV (31%). Levels I (5%) and V (6%) were rarely involved, and level V was never pathologically involved in the absence of nodal disease at other levels [188]. For patients in whom clinical nodal disease is evident on preoperative imaging, via nodal fine needle aspiration cytology, or at the time of surgery, surgical resection via comprehensive node dissection might reduce the risk of recurrence and, possibly, mortality. Some authors have attempted to evaluate the effectiveness of selective neck dissection in clinically N+ patients with laryngeal cancer [257-259]. Selective neck dissection (levels II–IV) could be considered for clinically N+ in some selected patients with supraglottic cancer. Selective neck dissection may be an appropriate treatment for patients with clinically N+ disease and nodal pathology limited to two or fewer neck levels [260].
D2-2. Management for clinically negative neck (N0) in patients with supraglottic cancer
Recommendation 19
(A) Elective ipsilateral neck dissection should be considered in patients with supraglottic cancer (weak recommendation, low-quality evidence).
(B) Elective contralateral neck dissection should be considered in patients with supraglottic cancer with T3/T4 primary tumors, midline crossing, clinically involved ipsilateral neck nodes, or suspicious extracapsular node extension (weak recommendation, low-quality evidence).
(C) Selective neck dissection of levels II, III, and IV is more appropriate than comprehensive neck dissection for patients with clinically N0 supraglottic cancer (strong recommendation, moderate-quality evidence).
The ideal management of the clinically N0 neck remains controversial. The main controversy surrounds the issue of whether steps should be taken to eradicate occult metastases in the clinically N0 neck. The incidence of nodal metastases varies widely from 10% to 50%, depending on the choices of clinical, imaging, and histopathological methods [207,261,262].
Elective ipsilateral neck dissection was previously recommended for all patients with supraglottic cancer [263,264]. The morbidity associated with selective neck dissection is very low. Djordjevic et al. [265] reported a statistically significant observed difference in the development of postoperative regional metastases in a prospective case-control study, with rates of 4.15% (eight cases) in the elective neck dissection group versus 11.8% (six cases) in the ‘wait and see’ group. Weber et al. [266] also showed a significant reduction in the incidence of cervical recurrent disease from 20% to 9%. These authors demonstrated that 38 of 39 recurrences among 202 patients with supraglottic cancer had developed in non-surgically treated necks. Redaelli de Zinis et al. [267] suggested elective neck dissection only for advanced-stage supraglottic cancers. In their study, occult metastases were observed in 0% of pT1, 26% of pT2, 46% of pT3, and 26% of pT4 cases. Some authors suggested considering elective neck dissection only when the tumor had spread into the vallecula, tongue base, or medial wall of the pyriform sinus, or when the tumor depth exceeded 1 mm [268-270]. However, the ‘wait and see’ policy has been proposed as an alternative to cN0 neck treatment in patients with supraglottic cancer [242,271-273]. In several studies, the neck disease-free survival rate did not differ significantly between patients with neck dissection and those subjected to a ‘wait and see’ evaluation [242,272,273]. In a study by Sessions et al. [242], survival rates of 75.5% and 79.9% were reported in the neck dissection and ‘wait and see’ groups, respectively; these rates were not significantly different.
Approximately 15% of the lymphatic circulation crosses the laryngeal midline and may lead to bilateral and contralateral metastases [274]. The contralateral undissected neck is the most common site of failure in cases of supraglottic cancer [275]. Lutz et al. [275] reported that the neck was the most common site for recurrent disease (39 of 47 patients), and such disease strongly tended to appear in the undissected, contralateral side (35 of 39 recurrent patients). Chiu et al. [276] also demonstrated that routine bilateral neck dissection reduced cervical recurrences and appeared to improve survival within the context of supraglottic cancer management. However, other authors preferred to perform ipsilateral neck dissection under the assumption of a higher risk of metastases according to the primary tumor site and extent or the ipsilateral nodal status [251,267,277]. Gallo et al. [207] demonstrated that supraglottic cancers involving or extending up to the midline had a higher risk of contralateral metastases when compared with well-lateralized tumors. Ozturkcan et al. [278] reported contralateral occult metastasis rates of 44% and 5% in patients with pathologically N+ and N0 ipsilateral necks, respectively. Routine bilateral neck dissection for the treatment of early-stage lateral supraglottic cancer with a clinically N0 neck might not be necessary because no significant improvements in regional control and survival have been observed with this technique relative to the use of ipsilateral neck dissection [272].
There is no general consensus regarding which type of neck dissection is more adequate in patients with cN0 supraglottic cancer. However, selective neck dissection of level II–IV has become the procedure of choice for the surgical treatment of clinically negative necks in patients with supraglottic cancer. In a prospective randomized trial that compared modified radical neck dissection with selective level II–IV neck dissection in 132 clinically N0 patients with supraglottic and transglottic carcinomas, no significant differences were observed in the incidence of neck recurrence (four in the modified radical neck dissection group and two in the selective neck dissection group) or survival (72.3% in the modified radical neck dissection group and 62.4% in the selective neck dissection group) [212]. Some authors showed that the level IIB and IV lymph nodes are rarely involved in cases of metastatic disease, and may be left in place during neck dissection in patients with N0 necks [215,279-281]. Superselective neck dissection to remove the level IIA and III lymph nodes could be considered for patients with SCC of the supraglottic larynx and a N0 neck [215,279,280].
E. Postoperative risk stratification/rehabilitation/long-term follow-up
E1. How we can stratify the risk of recurrence in postoperative laryngeal cancer patients? To which patients should postoperative adjuvant therapy be administered?
E1-1. Postoperative management and complications
Recommendation 20
Preoperative assessment and management of factors that predispose a patient to postoperative complications are necessary (strong recommendation, moderate-quality evidence).
Postoperative management includes the monitoring of vital signs, fluid and electrolyte balances, oxygenation, wound drainage, neck flap viability, and respiratory (e.g., tracheostomy tube care, airway humidification) and nutritional care. Erythema and edema of the skin flaps, fever, foul odor, and an elevated leukocyte count imply wound infection.
Pharyngocutaneous fistula may be suspected in a patient with a spiking fever and tense, warm, erythematous skin flap in the suprastomal region after total laryngectomy. Many investigators have reported factors that predispose patients to pharyngocutaneous fistula. Comorbidities such as diabetes, hepatic disease, thyroid disease, anemia, peripheral vascular disease, COPD, and malnutrition, the use of immunosuppressive medication, and local factors such as the tumor location and stage, persistent disease, pre- or postoperative radiotherapy, preoperative tracheostomy, extent of neck dissection, method of pharyngeal closure, and early nasogastric tube removal are considered predisposing factors for pharyngocutaneous fistula [282-289]. Recent metaanalyses have reported that COPD, a previous hemoglobin level of less than 12.5 g/dL, blood transfusion, previous radiotherapy or chemoradiotherapy, advanced primary tumor, supraglottic subsite, hypopharyngeal tumor site, positive surgical margins, and neck dissection were risk factors for fistula, whereas the suture material was not a significant factor [285,290].
Prolonged aspiration is a major morbidity experienced after partial laryngectomy. Usually, the incidence and severity of this condition are related to the extent of resection. Great care must be taken after SCL or SPL, and even after transoral endoscopic resection or total laryngectomy as aspiration may occur via tracheoesophageal puncture. Dysphagia may also occur after total and partial laryngectomy. Dysphagia after total laryngectomy is mainly due to stenosis of the neopharynx as a result of a tight surgical closure or cicatricial scar formation.
Stomal stenosis is a slowly progressive complication after total laryngectomy. Predisposing factors for this condition include the presence of a tube that induces local inflammation and fibrosis, postoperative radiotherapy, tracheoesophageal puncture prosthesis, or tumor characteristics. Local infection, female sex, and diabetes were found to correlate with stomal stenosis in several multivariate analyses [291-293]. This complication can be quite severe and may require surgical correction. Long-term use of a stomal tube may be necessary.
Hypothyroidism was reported in 13% to 38% of patients after laryngeal cancer treatment [294-296]. Surgeons should keep in mind that the risk of hypothyroidism may persist for several years. Therefore, thyroid function tests should be performed regularly after treatment completion [297]. Radiation therapy, thyroid gland invasion, nodal metastasis, and postoperative fistula were found to correlate significantly with the development of hypoparathyroidism [296].
E1-2. Adjuvant treatment
Recommendation 21
(A) Postoperative adjuvant treatment is recommended for stage III/IV laryngeal cancer (strong recommendation, high-quality evidence).
(B) Adjuvant radiation or chemoradiotherapy is recommended for patients with laryngeal cancer and risk factors such as a tumor with vascular invasion, perineural invasion, or multiple nodal metastases (strong recommendation, high-quality evidence).
(C) Adjuvant chemoradiotherapy is recommended for patients with laryngeal cancer and positive surgical margins or extracapsular nodal extension (strong recommendation, high-quality evidence).
Given the poor prognosis of stage III/IV HNSCC, a combination of radical surgery and postoperative radiation therapy has remained the standard treatment. Generally, a total dose of 60 to 66 Gy of conventional postoperative radiation is administered for resectable locally advanced HNSCC [298]. Radiotherapy should be initiated within 6 weeks after surgery [299]. However, local recurrence and distant failure rates are as high as 30% and 25% and the 5-year survival rate is as low as 40% after radical surgery with postoperative radiotherapy. Furthermore, pathologic findings of surgical specimen such as a positive surgical margin, vascular invasion, and extracapsular nodal extension (ECE) adversely affect the prognosis.
Several randomized trials have been conducted to clarify the role of adjuvant chemoradiotherapy in the postoperative management of patients with advanced resectable HNSCC and poor prognostic factors. The RTOG 9501 study included patients with advanced HNSCC and high risk factors, including multiple lymph node metastases (≥2), ECE, or microscopic surgical margin involvement. This study found that the chemoradiotherapy group had a higher locoregional control rate (82% vs. 72% for radiotherapy) and improved disease-free survival (hazard ratio for disease or death, 0.78 relative to radiotherapy; 95% CI, 0.61 to 0.99; P=0.04). However, overall survival was not significantly different (hazard ratio for death, 0.84; 95% CI, 0.65 to 1.09; P=0.19) [300]. Concurrently, the European Organization for Research and Treatment of Cancer (EORTC) suggested that stage III/IV disease, perineural infiltration, vascular tumor embolism in addition to ECE, and microscopic surgical margin involvement indicate candidates for chemoradiotherapy (EORTC 22931) [301]. That trial recruited 338 HNSCC patients with adverse features and found that the chemoradiotherapy group, when compared with the radiotherapy group, had better 5-year progression-free survival (47% vs. 36%) and overall survival rates (53% vs. 40%) and a lower recurrence rate (18% vs. 31%). To identify the most suitable patients for chemoradiotherapy, data from the two studies were subjected to a combined analysis [302]. Accordingly, ECE and microscopic margin involvement were found to be the most significant prognostic factors for loco-regional recurrence and survival. In contrast, chemoradiotherapy yielded no advantage over radiotherapy alone in patients with multiple lymph node metastases but without ECE. Long-term follow-up data from the RTOG 9501 trial also demonstrated the significance of ECE and microscopic margin involvement [303]. Among patients in that study with HNSCC and ECE or microscopic margin involvement, the chemoradiotherapy group had a lower 10-year locoregional recurrence rate (21.0% vs. 33.1%) and higher 10-year disease-free survival (18.4% vs. 12.3%) and overall survival rates (27.1% vs. 19.6%). However, there were no additional gains in locoregional control and disease-specific survival in the chemoradiotherapy group when compared with the radiotherapy only group among patients with multiple lymph node metastases.
E2. Postoperatively, what types of rehabilitation and/or psychiatric support are required for patients with laryngeal cancer?
E2-1. Swallowing rehabilitation
Recommendation 22
(A) Swallowing rehabilitation can be recommended for patients with aspiration tendencies after transoral surgery or open partial laryngectomy (strong recommendation, moderate quality evidence).
(B) A modified barium swallow with videofluoroscopy can be recommended for an evaluation of swallowing function (strong recommendation, low-quality evidence).
Laryngeal cancer patients often develop swallowing disorders that delay patient recovery after radiation therapy or laryngectomy; these disorders are often life-threatening.
In general, patients who undergo hemilaryngectomy have a relatively lower rate of aspiration and a more rapid return to a normal diet, compared to patients who undergo supraglottic laryngectomy [304,305]. However, a broader dissection site, which may include arytenoid cartilage, increases the risk of developing aspiration [306,307]. A brief change in swallowing, particularly liquids, will occur after surgery; within 1 to 2 weeks; however, recovery begins as the normal side of the larynx compensates for the damaged side [308].
Up to 74% of patients who underwent supraglottic laryngectomy reported postoperative aspiration, and approximately 4 to 6 weeks were required for these patients to achieve a safe and effective oral intake [308,309]. However, a longer period might be needed to recover normal swallowing once a large tongue base resection has been performed [304].
Although a high incidence of dysphagia was observed among patients who underwent SCL, good swallowing recovery rates were observed at 3 months after surgery [310]. Several studies observed better functional results after SCL-CHEP than after SCL-CHP [311-313].
Patients experience decreased pharyngeal wall contraction and pharyngoesophageal pressure after total laryngectomy, and these conditions affect the swallowing pattern; however, patients may return to a normal diet within a month [314].
Radiotherapy to the pharynx and larynx can damage the pharyngeal constrictor and trigger dysphagia. Fibrosis in the irradiated tissues can lead to dysfunctional movement in the oral tongue, tongue base, pharyngeal constrictor muscles, and larynx [315].
Useful diagnostic tests for dysphagia include the modified barium swallowing procedure under videofluorography and the swallowing examination via fiberoptic endoscopy. Of these, the modified barium swallow provides much of the information necessary to develop a swallowing rehabilitation plan [316-320].
Swallowing rehabilitation methods after treatment for laryngeal cancer include changes in the head or body posture, swallowing maneuvers, and modifications of the bolus size or consistency [321-323]. Changes in head or body postures, such as chin down, head back, head rotation, and lateral head tilt postures, are used in controlling the bolus flow and also in reducing or eliminating aspirations. The chin down posture carried out either alone or along with other postures or maneuvers, has been reported to yield successful results in postsurgical patients with head and neck cancer; specifically, aspiration was decreased or eliminated in 50% of patients with tongue base resection and 90% of patients with oral or laryngeal resection [321,324]. The head rotation posture induces compensatory movements in the healthy side of the arytenoid, which might effectively reduce aspiration in posthemilaryngectomy patients experiencing difficulties in closure of remaining vocal fold [325].
Swallowing maneuvers include the supraglottic swallow and super-supraglottic swallow maneuvers, effortful swallow maneuver, Mendelsohn maneuver, and tongue hold method [326,327]. Among the various methods, the supraglottic swallow and super-supraglottic swallow maneuvers are especially effective not only for reducing aspirations in supraglottic laryngectomy patients but also in patients who have received a full course of radiotherapy for head and neck [308,328].
Surgical excision of laryngeal cancer may have a significant impact on the swallowing function because of the following factors: tumor site, resected structures, and subsequent reconstruction. Dysphagia also occurs after radiation therapy. Therefore, diagnostic imaging procedures such as the modified barium swallow with videofluorography should be used along with various rehabilitation methods for successful swallowing rehabilitation.
E2-2. Voice rehabilitation methods after total laryngectomy
Recommendation 23
Options for voice rehabilitation, including esophageal speech, electrolarynx, and tracheoesophageal speech with a voice prosthesis, should be offered to patients who have undergone total laryngectomy (strong recommendation, low-quality evidence).
The loss of the laryngeal voice is the main consequence of total laryngectomy; accordingly, learning to use a new voice is the main objective of rehabilitation for these patients.
Commonly used voice rehabilitation methods include esophageal speech, electrolarynx usage, and tracheoesophageal puncture for tracheoesophageal speech, which can be performed primarily or secondarily [329].
There are distinctive advantages to using the esophageal voice method; for example, the patient’s hands remain free and the costs of the surgical procedure and/or a speaking device are not required. The acquisition of esophageal speech, however, requires 30 to 50 hours of intense speech therapy [330]. Furthermore, the rehabilitation success rate varies depending on the individual conditions [331]. Compared with lung-powered speech, patients can only speak short phrases and may not be satisfied with the voice quality [331]. The resulting voice is rough and breathy, with a low pitch and reduced loudness [332,333].
The electrolarynx method uses electromagnetically generated sound-producing vibrations; however, the substitute voice is monotonous and mechanical [332,334]. The electrolaryngeal voice can be used when other voice rehabilitation methods have failed, or even if other options are available [330,335-337]. According to related studies, more than 50% of patients who undergo total laryngectomy will continue to rely on the electrolarynx as their primary method of verbal communication at 2 years after surgery [338].
Recently, the use of tracheoesophageal speech vocalization with a voice prosthesis has increased among patients who have undergone total laryngectomy for laryngeal cancer [339]. The Provox voice (Atos Medical, Milwaukee, WI, USA) prosthesis, which was developed by the Netherlands Cancer Institute in 1988, is currently of the most widely used devices [340,341]. In several studies, tracheoesophageal speech with a voice prosthesis yields speech that is considered more normal than esophageal speech [342,343]. In addition, functional outcome analyses have found that tracheoesophageal speech with a voice prosthesis yields a good voice quality [344,345]. Voice prostheses may be inserted either at the time of total laryngectomy (primary) or at a later stage (secondary). A primary prosthesis provides almost immediate and satisfactory voice rehabilitation [346,347].
E2-3. Shoulder dysfunction after neck dissection
Recommendation 24
(A) The spinal accessory nerve should be identified during neck dissection (strong recommendation, moderate-quality evidence).
(B) Early shoulder rehabilitation is recommended after surgery (strong recommendation, moderate-quality evidence).
General complaints and functional impairment of shoulder are common sequelae after neck dissection. These complications may be attributable not only to nerve injury caused by traction or other surgical procedures but also to secondary effects such as adhesive capsulitis or myofascial pain [348]. Shoulder problems gained after the neck dissection are caused by the dysfunction of spinal accessory nerve. In addition, the secondary glenohumeral stiffness can be caused by weakness of the scapulohumeral girdle muscles and also by lack of postoperative mobility [349].
The accessory nerve can be found in levels II and V during neck dissection. In level V, the spinal accessory nerve is more superficial. Thus it is easy to be led to local iatrogenic surgical trauma or inadvertent division of the nerve [350].
Injury to the spinal accessory nerve, which provides motor innervation to the sternocleidomastoid and trapezius, results in pain, losses of mobility and strength, and deformity of the shoulder homolateral to the dissection [351,352].
Even if the spinal accessory nerve has not been injured, shoulder complaints can be detected commonly after the neck dissection. Several studies revealed that 31% to 60% of patients after modified radical neck dissection, and 29% to 39% of patients after selective neck dissection are found to be experiencing shoulder related symptoms [353,354].
Spinal accessory nerve sparing during neck dissection is associated with a significant reduction in long-term shoulder disability among 5-year survivors of head and neck cancer [355]. A number of studies have demonstrated that spinal accessory nerve-preserving neck dissection is associated with reduced shoulder pain, better shoulder function, and an improved overall quality of life, compared to radical neck dissection [356-360].
In addition, several studies have reported that neck dissection with level 2b preservation reduces spinal accessory nerve trauma [361]. During surgery, spinal accessory nerve neuromonitoring may be used to predict a patient’s postoperative shoulder function and activity restrictions [362].
Physical therapy is essential in dealing with shoulder complaints after neck dissection whether the spinal accessory nerve has been preserved or sacrificed [348]. This type of therapy is aimed at an early recovery of passive motion, and has been shown to be beneficial in preventing the occurrence of joint fibrosis. Physical therapy is very important for promoting functions and for reducing pains. This can be done by maintaining the lengths of muscles and ranges of movement and also by preventing secondary complications such as adhesive capsulitis [363]. In addition, several reports have recommended the early repair of iatrogenic spinal accessory nerve damage to avoid significant atrophy of the trapezius muscle and long-term functional deficits [364,365].
Progressive resistance exercise training, which can be done along with the standard physiotherapy, may improve scapular stability and strength of the upper extremity [366,367]. Moreover, physical therapy was found to have a significant positive effect on the patient’s quality of life after neck dissection [368].
The importance of a timely initiation of physical therapy has also been supported by epidemiologic studies of the clinical course of neck and shoulder symptoms after presentation. A Dutch study reported a low recovery rate after consultation for shoulder symptoms; 24% of patients reported recovery at their 3-month follow-up examinations, and 32% reported recovery at their 12-month follow-up examinations. Therefore, a timely initiation of physical therapy after neck dissection appears to be important because it is more difficult to treat already established shoulder complaints and disabilities [348].
E2-4. Counseling for smoking cessation
Recommendation 25
Smoking cessation from the time of diagnosis is strongly recommended for patients with laryngeal cancer (strong recommendation, high-quality evidence).
In general, smokers have higher infection and pulmonary complication rates. In addition, smokers have relatively longer postoperative hospital stays, compared with non-smokers [369,370]. Smoking leads to increases in all-cause mortality, cancer-specific mortality, and the risk of a second primary cancer. Furthermore, smoking is known to correlate with an increased rate of cancer recurrence, poor treatment responses, and increased treatment-related toxicity [371].
The risk of wound complications after reconstructive head and neck surgery is closely related to serum cotinine concentration [372]. Among patients receiving radiotherapy for head and neck cancer, smokers had a poorer locoregional control rate [373,374]. Patients with head and neck cancer who continue to smoke throughout radiotherapy experience relatively poorer therapeutic effects and a shorter survival time, compared with non-smoking patients and those who quit smoking before treatment [375].
Smoking affects the cytochrome P450 enzyme, which ultimately impacts the metabolism of chemotherapeutic and targeted therapeutic agents; specifically, the drug clearance times and plasma concentrations deviate from the normal values [376-378].
Smoking cessation immediately reduces the blood carbon monoxide level and respiratory irritation, and improves lung function. Over the long term, smoking cessation significantly reduces the incidence of smoking-related diseases and mortality [379]. Smoking cessation at or near the time of a cancer diagnosis reduces the risk of therapy-related complications and decreases the rate of second primary cancer onset, compared to smoking continuation [380-382]. Therefore, smokers with cancer must be educated about the specific risks of smoking during their particular anti-cancer treatments; specifically, smoking cessation before cancer treatment initiation would be the best option, if possible.
Pharmacotherapy is most effective when combined with behavioral therapy [383-385]. The recommended initial treatment durations are 12 weeks for varenicline and combination nicotine replacement therapy, and 7 to 12 weeks for bupropion [386]. Successful behavior therapy strategies employ practical counseling, which addresses problem solving and skill training, as well as social support and motivational interviewing [387].
E2-5. Psychiatric consultation
Recommendation 26
Psychiatric consultation should be considered for the patients with laryngeal cancer (strong recommendation, high-quality evidence).
The diagnosis and subsequent treatment of head and neck cancer could have potentially devastating impacts on psychosocial functioning [388].
Cancer-related symptoms such as fatigue, pain, anxiety, and depression frequently interfere with patient’s activities of daily life [389-391]. A study of more than 5,000 patients found that 6% of patients with cancer experienced suicidal ideation [392]. Patients with uncontrolled mood and adjustment disorders have a high tendency to suicide [393-395]. Older patients and male patients with head and neck cancer or myeloma are reported to be at a higher risk of committing suicide [396].
Compared to those who have undergone partial laryngectomy, patients who have undergone total laryngectomy are known to experience more severe psychiatric stress as a result of permanent voice impairment and a reduced life expectancy [397,398]. A study of 74 patients subjected to total laryngectomy reported a significant degree of abnormal findings such as sexual dysfunction, depression, and decreased self-esteem [399].
Psychiatric mood disorders, such as depression, are usually managed with psychotherapy or psychotropic medication [400-406]. Otherwise, referrals to social work counseling and chaplaincy services could be considered. Patients who endanger themselves or the others should be considered for psychiatric consultation. These patients need close and increased monitoring and any dangerous objects near them should be removed. Psychiatric treatment and hospitalization can be considered if necessary [407].
E3. How can we postoperatively follow-up patients with laryngeal cancer?
E3-1. Long-term follow-up schedule
Recommendation 27
(A) Patients should be regularly examined for more than 5 years after treatment (strong recommendation, high-quality evidence).
(B) Patients should be followed up frequently during the first 2 years because of the high risk of locoregional recurrence; this schedule includes every 1 to 3 months during year 1, and every 2 to 6 months during year 2 (strong recommendation, low-quality evidence).
There are several reasons to subject patients with laryngeal cancer to a posttreatment follow-up, including the early identification of recurrent disease, early detection of new primary tumors, monitoring and management of complications, optimization of rehabilitation, promoting cessation of smoking and excessive alcohol consumption, providing support to patients and their families, and patient counseling and education.
Frequent posttreatment visits should be recommended to patients with head and neck cancer, including laryngeal cancer, especially during the first 2 years when the risk of locoregional recurrence is known to be high; the visit frequency may be reduced thereafter, and follow-up can be completed by year 5. Patients with high-risk disease or specific tumors, those who require continuous special rehabilitation, and those who prefer a longer period of follow-up may be examined for a longer period of time, and even the remainder of their lives [408-417].
The European Journal of Surgical Oncology advised a follow-up schedule comprising visits every 4 to 6 weeks during the first 2 years, every 3 months during year 3, twice yearly in years 4 and 5, and yearly thereafter [418].
Members of the American Society for Head and Neck Surgery reported 73% agreement in response to a schedule comprising monthly follow-up visits during the first year after surgery, visits every 2 to 3 months during year 2, and visits every 4 to 6 months during years 3 to 5 years after surgery [419].
The National Comprehensive Cancer Network guideline also recommends follow-up visits every 1 to 3 months during year 1, every 2 to 6 months during year 2, every 4 to 8 months during years 3 to 5, and annual follow-ups thereafter [420].
Many studies have shown that the first 2-year follow-up generally occurs between 4 and 8 weeks postoperatively, and subsequent visits occur every 3 to 6 months [408-413].
E3-2. Tests during the follow-up period
Recommendation 28
(A) Laryngoscopic examinations should be performed regularly to check for local recurrence (strong recommendation, low-quality evidence).
(B) A CT or MR study is recommended within 6 months after treatment to provide baseline images for later reference (strong recommendation, low-quality evidence).
(C) PET-CT is recommended for the detection of distant metastasis, recurrence, and second primary tumors (strong recommendation, moderate-quality evidence).
(D) A chest radiography or CT study is recommended for the detection of lung metastasis and second primary tumors in the lung (strong recommendation, moderate-quality evidence).
(E) US can be recommended for the detection of cervical lymph node recurrence (weak recommendation, low-quality evidence).
The first step in the posttreatment follow-up of a patient with laryngeal cancer involves educating the patient about the potential symptoms and signs of recurrence. This education should include tobacco smoking and alcohol cessation programs [409, 421,422].
During follow-up, a rigid telescope, transnasal video, or fibroscopy should be used for laryngeal inspection, and the neck should be palpated. According to the research, laryngoscopy and stroboscopy provide better accuracy (100% for both methods) than history taking and physical examination (33%) [10]. The use of a videostroboscope can provide valuable additional information [423].
Chest radiography is performed as a part of routine a head and neck cancer follow-up to detect lung metastasis and second primary tumors in the lung. According to de Visscher and Manni [424], yearly performed chest radiographies were only useful in patients with laryngeal index tumors; the incidence of secondary primary and metastatic tumors was higher in supraglottic cancer than in glottic cancer. Chest CT should be used instead of chest radiography to screen patients with advanced HNSCC [424-426].
Research conducted to evaluate how efficient the use of US and palpation are during follow-up revealed that US showed 97.5% of accuracy in successfully detecting enlarged lymph nodes, with an accuracy of 97.5% [427]. Other researchers have also reported that US and US-guided fine needle aspiration cytology provide information critical to the detection of cervical lymph node recurrences [428-430].
Baseline CT or MR, conducted between 3 to 6 months after the surgical, radiological, or combined treatment of high-risk HNC, can be compared with subsequent images for the earlier detection of abnormalities [431,432]. Patients who have undergone radiotherapy for laryngeal cancer require careful follow-up studies involving clinical examinations and CT imaging at 3- to 4-month intervals for a duration of 2 years after radiotherapy [432].
MR and PET-CT scanning exhibit superior performance for the detection of recurrences and second primary tumors. PET-CT is also advantageous as a systematic evaluation and has a reported sensitivity of 92% in detecting recurrent laryngeal cancer. PET-CT shows nearly 100% of accuracy in diagnosing distant metastasis in cancer patients [433-436].
In patients treated with primary radiation therapy alone, CT, MR and US cannot specifically differentiate postradiation edema from recurrence. Therefore, CT, MR, or PET-CT to obtain baseline images for later reference should be performed 3 to 6 months after treatment [434].
Patients who have undergone extended resection via transoral laser surgery require regular laryngeal examinations every 4 to 8 weeks during the first year after surgery, as the risk of locoregional recurrence remains high [408]. Second look microlaryngoscopy is still considered somewhat controversial, but may be adapted for uncertain (close or altered for iatrogenic artifacts) surgical margins, granulomas, web formation, other postexcision abnormal tissue growth at the level of the primary resection site (despite appropriate medical and voice therapy), or the involvement of certain laryngeal subsites (anterior commissure, ventricle, subglottis) [437-439].
For patients who have undergone open partial laryngectomy, a clinical laryngoscopic examination and CT scan of the primary site are recommended [408]. Although the optimal follow-up regimen after total or pharyngolaryngectomy remains under dispute, a clinical examination of the remaining upper aerodigestive track and neck should be performed, followed by contrast CT if the result is positive [409]. Follow-up PET-CT has been incorporated to screen for metastasis in patients who have undergone surgery accompanied by radiotherapy or chemoradiotherapy [434,435,440-442].
Patients treated with definitive chemoradiotherapy should undergo PET-CT at 3 months (12 weeks) after the completion of therapy to assess the primary and neck disease response and to plan salvage neck surgery if required [434,435,440-442].
NBI, which uses pathognomonic neoangiogenic patterns to detect abnormal lesions, reportedly has a true-positive laryngeal cancer lesion detection rate that is 18% higher than that of conventional white light endoscopy. Furthermore, NBI features both high accuracy as well as the ability to differentially diagnose abnormal regions from postradiotherapy or chemoradiotherapy inflammatory and/or cicatricial changes [443-445].
Tumor markers and gene expression profiling, which are poorly sensitive and have low cost-to-benefit ratios, have yet to be proven useful for the follow-up of laryngeal cancer [446,447].
E3-3. Thyroid function evaluation
Recommendation 29
(A) A thyroid function evaluation is recommended to evaluate the presence of hypothyroidism in patients with laryngeal cancer who have undergone head and neck radiation therapy or thyroid gland removal (partial or full) (strong recommendation, low-quality evidence).
(B) Thyroid function should be evaluated twice yearly during the first 5 years after treatment, and annually thereafter. Thyroid function may be subjected to periodic follow-up evaluation for 10 years (weak recommendation, low-quality evidence).
Head and neck irradiation results in biochemical hypothyroidism in at least 50% of patients. Moreover, a definitive initial surgery that removes part of the thyroid gland can increase the risk of hypothyroidism [448]. Previous studies revealed that 10% to 70% of cases after head and neck cancer treatment suffer from thyroid dysfunction [294,449-451].
Thyroid function is determined by measuring the serum levels of thyroid stimulating hormone (TSH) and free thyroxine (FT4). Thyroid function is classified into three categories: firstly, euthyroidism, which is with normal TSH and FT4 levels; secondly, subclinical hypothyroidism, which has increased TSH and normal FT4 levels; and lastly, clinical hypothyroidism, which shows increased TSH and decreased FT4 levels [452].
Several pathophysiologic mechanisms, such as vascular supply, may give rise to hypothyroidism. It is because the vascular structure near the thyroid might be iatrogenically damaged or intentionally sacrificed during the course of neck dissection, which thus affect the blood supply and eventually the function of the thyroid. Furthermore, the thyroid gland itself may be subjected to partial or full resection for oncologic reasons. Radiotherapy-induced fibrosis may result in decrease of thyroid function not only be compromising the thyroid vascularity but also by causing fibrosis of the whole gland [294].
Increased TSH levels have been detected in 20% to 25% of patients who have received neck irradiation; accordingly, these patients are at an increased risk of hypothyroidism [453]. In a retrospective review of 147 total laryngectomy patients, 19.9% of patients developed hypothyroidism at year 3 of follow-up; at years 6 and 10, 38.6% and 93.3% had developed hypothyroidism, respectively [454]. Such reports support regular thyroid evaluations for a period of at least 10 years after receiving treatment for laryngeal cancer.
In conclusion, thyroid dysfunction is a frequently occurred complication in up to 50% of patients who have undergone laryngectomy and radiotherapy but tend to be unrecognized easily. Therefore, a regular thyroid function tests are recommended after treatment for laryngeal cancer [452].
F. Salvage surgery
F1. What is the appropriate surgery for recurrent laryngeal cancer?
F1-1. Salvage surgery for a local failure of non-surgical treatment
Recommendation 30
(A) Total laryngectomy is recommended for recurrent T3/T4 cancer (strong recommendation, low-quality evidence).
(B) Transoral laser microsurgery can be used as a salvage option for recurrent T1/T2 cancer (weak recommendation, low-quality evidence).
(C) Open partial laryngectomy, especially supracricoid laryngectomy, can be recommended for recurrent T2 and selected T3 cancers (weak recommendation, low-quality evidence).
(D) Pectoralis major muscle flap onlay reinforcement may reduce fistula formation resulting from salvage total laryngectomy after concurrent chemoradiotherapy; however, the panels cannot recommend for or against routine provision of this procedure (No recommendation, insufficient evidence).
The use of salvage surgery for residual or recurrent cancer after non-surgical treatment has increased following the acceptance of organ preservation into mainstream laryngeal cancer treatment strategies. The reported recurrence rate after radiotherapy alone ranges from 32% to 58% [455-457]. Among early glottic cancers, the recurrence rate after radiotherapy ranges from 10.4% to 32% [456,458-462]. Among advanced cancers, concurrent chemoradiotherapy significantly decreases the incidence of locoregional failure; in the RTOG 91-11 trial, only 16% of patients required salvage total laryngectomy after concurrent chemoradiotherapy [463].
Total laryngectomy remains the mainstay of salvage treatment after radiotherapy, with or without chemotherapy, because many patients (up to 56% to 59%) present with more advanced-stage disease after radiotherapy [464,465]; in addition, there exists some concern about submucosal spread in cases of radiation failure [466]. The rate of total laryngectomy after radiation failure ranged from 44.8% to 92%, even for an initially early glottic cancer [461,464,465,467-470]. However, laryngeal preservation surgical techniques have improved, and good survival results have been reported. Vertical partial laryngectomy, frontolateral laryngectomy, supraglottic laryngectomy and, more recently, transoral laser microsurgery or SCL are frequently used laryngeal saving techniques used for salvage surgery [407,471-476]. The general contraindications for laryngeal preserving surgery may include the following: (1) arytenoid fixation; (2) invasion of the posterior commissure; (3) subglottic extension of more than 5 mm posteriorly and 5 to 10 mm anteriorly or to the upper border of the cricoid cartilage; (4) cricoid cartilage invasion and major thyroid cartilage invasion (T4); (5) massive pre-epiglottic space involvement; (6) positive margins in a frozen section; and (7) extralaryngeal spread. Transoral laser microsurgery may be preferred if the recurrent disease does not extend beyond the original site and the larynx is mobile. However, if the recurrent tumor has extended beyond its original site, has impaired vocal cord motion or caused fixation, and/or presents with pre-epiglottic space or thyroid cartilage invasion, then SCL should be strongly advocated [466,477].
The reported local control rate of transoral laser microsurgery ranges approximately from 57% to 65%, whereas that of external laryngeal preservation surgery, including SCL, ranges from 77% to 85% [473,478-480]. For rT1 or rT2 lesions, approximately 42% to 70.6% of patients can be treated with a single transoral laser surgical procedure [481-485]. In addition, the laryngeal preservation rate of transoral laser microsurgery ranges from 62.3% to 86% [483,484]. In one report, transoral laser microsurgery yielded good local control for rT1 lesions (87.5%), but unsatisfactory outcomes for rT2 lesions (16.6%). However, that report did not observe differences in overall or disease-specific survival after the second salvage [482]. In contrast, Steiner et al. [485] reported the use of transoral laser microsurgery in patients with rT4 disease. In other words, the surgeon’s level of expertise seems to be an important factor in laser microsurgery.
Complications of open surgery, including total laryngectomy, increase significantly after concurrent chemoradiotherapy, and the reported local complication rates range from 45% to 92% [486,487]. In particular, the risk of fistula development increases (23.5% to 68%) [486,488,489]. Accordingly, procedures such as a pectoralis major muscle flap overlay or the uses of other fresh tissues (e.g., free flap) have been attempted to prevent fistula formation. However, the data are not consistent. Some papers have reported a similar fistula rate even with a pectoralis major muscle flap; however, the effect was the prevention of large fistulas, thus reducing the rate of reoperation [420,490,491]. Others have reported the usefulness of a pectoralis major muscle flap for fistula prevention. These researchers reported that the fistula rate associated with primary closure (31% to 58%) could be reduced by using a pectoralis major muscle flap onlay reinforcement (10.5% to 22%) [492-497]. Therefore, pectoralis major muscle flap onlay reinforcement may play a role in fistula prevention, but the effect is remained uncertain.
F1-2. Management of the N0 neck during salvage surgery after nonsurgical treatment
Recommendation 31
(A) Ipsilateral elective neck dissection is recommended for recurrent supraglottic, transglottic, or rT3/rT4 glottic cancer (strong recommendation, low-quality evidence).
(B) Bilateral elective neck dissection can be considered for recurrent supraglottic cancer (weak recommendation, low-quality evidence).
(C) Elective neck dissection can be avoided for recurrent glottic rT1N0/rT2N0 cancer with initial N0 (weak recommendation, low-quality evidence).
Comprehensive neck dissection is recommended for regional failure, with a reported survival rate of approximately 61.2% [498]. Elective neck dissection is generally recommended for the salvage treatment of supraglottic or transglottic cancer, given the high rate of occult metastasis with these tumors (28% to 60%) [478-480,499,500]. In addition, elective neck dissection is considered suitable for advanced recurrent glottic cancer; a previous report described improved disease-free and overall survival in patients with locally advanced disease who had undergone elective neck dissection, but not in patients with limited disease [501]. Among patients with rT3 or higher disease, the reported occult metastasis rate is approximately 20%. Very few reports have discussed the rate of occult metastasis in the contralateral neck, and therefore it is difficult to draw conclusions regarding the need for elective neck dissection. Occult contralateral neck metastasis has been found in 0% to 6.4% of laryngeal cancer cases [502,503]. Accordingly, bilateral neck dissection at the time of laryngectomy cannot be recommended generally. However, for recurrent supraglottic cancers, the occult bilateral neck metastasis rate was as high as 15% in one report, and the authors recommended bilateral elective neck dissection for such patients [504].
However, the issue of elective neck dissection during salvage open laryngectomy remains controversial, especially for cases of early recurrent glottic cancer. The initial N stage before radiation therapy may correlate with occult metastasis during salvage surgery. The reported occult metastasis rates in initially N0 necks range from 7.7% to 10%, whereas the corresponding rate in initially N+ necks is 50% [503,504]. Other studies reported low occult metastasis rates (4% to 5%) but high complication rates after elective neck dissection, with overall complication rates of 42.2% in the neck dissection group and 21.3% in observation group, and corresponding fistula rates of 32% to 57.2% and 13.4% to 18%, respectively [505,506]. Furthermore, the complication rate increased to as high as 67% in cases involving bilateral neck dissection [507]. Therefore, the researchers concluded that the benefit of elective neck dissection, especially bilateral neck dissection, should be balanced against the increased risk of morbidity. Another paper that investigated the role of preoperative CT scanning suggested that among N0 patients the preoperative CT metastasis rate was only 3% [508]. Given these data, observation of the neck is suggested for some patients with rT1 or rT2 and clinically rN0 disease [509].
F1-3. Salvage surgery for recurrence after surgical therapy
Recommendation 32
In eligible cases, extensive resection is recommended for a stomal recurrence after total laryngectomy (weak recommendation, low-quality evidence).
The reported recurrence rates after initial transoral laser microsurgery were 13% for T1 disease and 15.4% for T2 [109]. Furthermore, the reported total laryngectomy rates among patients with recurrent disease after transoral laser microsurgery range from 40.9% to 45% [109,510]. However, other papers suggest repeated transoral microsurgery as a treatment option, with reported 5-year survival rates of 75.1% among early recurrent cases and 51.6% among advanced cases [511].
However, recurrence after an initial total laryngectomy was associated with a poor prognosis, and only 21% to 27.5% of such cases were eligible for surgical salvage [512,513]. Particularly for stomal recurrence, the reported overall 2-year survival rate ranges from 10% to 16% with a median survival range of 6 to 11 months, even after surgical salvage treatment [514-516]. The reported rates of stomal recurrence range from 1.2% to 10.8%, according to the literature [514,515,517-521].
Extensive studies of the risk factors for stomal recurrence have been conducted. Preoperative tracheostomy, subglottic involvement extent, advanced tumor, and paratracheal node metastasis were reported to correlate positively with stomal recurrence [515,519,521-531]. However, the risk of preoperative tracheostomy for stomal recurrence is controversial, with some papers reporting negative results [532,533].
Radiotherapy and chemotherapy provide only limited palliation, whereas extensive resection offers the best chance of a cure [523].
HIGHLIGHTS
▪ Korean Society of Thyroid-Head and Neck Surgery (KSTHNS) developed the practice guideline about surgical treatment of laryngeal cancer.
▪ The multidisciplinary team approach is important in decision of laryngeal cancer patients.
▪ This guideline starts with the assumption that the surgery is decided as the treatment option.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
We appreciate the external consultation provided by experienced surgeons (Kwang Hyun Kim, Seoul National University; Nam Yong Do, Chosun University; Myung Whun Sung, Seoul National University; Phil Sang Chung, Dankook University; Jin Ho Son, Kyungpook University; Young Sam Yoo, Inje University; and Si Youn Song, Youngnam University) who provided expert opinion.
Notes
ENDORSEMENT FROM OTHER SOCIETIES
The final manuscript was endorsed by the boards of executives of the Korean Cancer Study Group (KCSG), Korean Society for Head Neck Oncology (KSHNO), Korean Society of Laryngology, Phoniatrics and Logopedics (KSLPL), and Korean Society of Otorhinolaryngology-Head and Neck Surgery (KORL).
Supplementary Material
Search key words and recall ratio
List of references which used to induce recommendations
Delphi questionnaire for recommendations in laryngeal cancer surgery guideline