Increased Risk of Psychopathological Abnormalities in Subjects With Unilateral Hearing Loss: A Cross-Sectional Study
Article information
Abstract
Objectives
Although unilateral hearing loss (UHL) has been proven to be associated with educational and behavioral problems, few studies have investigated psychopathological abnormalities in this population. The aim of this study was to evaluate the psychopathological influence of UHL among Korean 19-year-old males.
Methods
The authors retrospectively compared the objective personality test profiles of 602 subjects with UHL with those of 23,790 peers with normal hearing. All participants in the current study were 19-year-old males who underwent a physical examination and completed the Korean Military Multiphasic Personality Inventory for conscription at the Military Manpower Administration from February 2015 to December 2016.
Results
Significantly higher scores were found on neurosis scales in the UHL group than in the normal-hearing group (50.9± 10.8 vs. 44.9±6.0 for anxiety; 51.0±10.5 vs. 44.9±5.2 for depression; 51.1±10.4 vs. 45.1±6.81 for somatization, all P<0.001). The psychopathy scales were also significantly higher in the UHL group than in the normal-hearing group (49.3±9.4 vs. 46.3±5.7 for schizophrenia; 51.1±11.2 vs. 44.3±5.8 for personality disorders; 51.1±10.5 vs. 45.7±3.7 for paranoia, all P<0.001).
Conclusion
Nineteen-year-old males with UHL tended to have more abnormal results on personality tests than controls with normal hearing, suggesting that UHL may be related with a higher risk of psychopathology.
INTRODUCTION
Unilateral hearing loss (UHL), which refers to the coexistence of a hearing impairment in one ear with normal hearing in the other, affects 0.5–1 per 1,000 newborns [1,2]. Due to delayed-onset congenital UHL and acquired UHL, the prevalence increases to 3%–6% at school age, and 7.9% of U.S. adults are reported to have UHL [3,4]. Temporal bone anomalies such as an enlarged vestibular aqueduct and cochlear nerve deficiency are found in 28.9%–50% of UHL patients, and other etiologies including congenital cytomegalovirus infections, meningitis, and trauma may be associated with UHL [5-8].
In the past, UHL was thought to be a disease entity of negligible importance because subjects with UHL were thought to experience few, if any, communication or educational problems. However, since the 1980s, several studies have shown that UHL is associated with a higher rate of educational and behavioral problems, which may require repeating grades or additional educational assistance [9,10]. Studies have also demonstrated that school-aged children with UHL have a higher risk of delays in speech and language development than their peers with normal hearing [11,12]. Moreover, behavioral and functional imaging studies have indicated that UHL may have negative effects on cognition and executive function [13,14]. In this regard, the importance of UHL in terms of speech-language development, academic performance, and cognitive function has now been widely accepted.
Despite this recognition, the impact of UHL on quality of life and, more specifically, on psychopathological abnormalities has not been thoroughly studied. Using quality-of-life–related questionnaires or hearing handicap inventories, a few recent studies have suggested that UHL may be associated with emotional and social-situational problems [15,16]. However, the psychopathological impact of UHL is poorly understood, since studies with a large number of subjects using standardized psychometric tests of personality and psychopathology such as the Minnesota Multiphasic Personality Inventory (MMPI) are scarce.
Previous studies have reported that bilateral hearing-impaired children are extremely vulnerable to poor psychosocial development and have higher levels of psychological distress than their normal-hearing peers [17,18]. Similarly, we hypothesized that UHL could also pose a risk of psychopathological problems, and this paper reports a retrospective cross-sectional study that was conducted to evaluate whether this hypothesis is true.
MATERIALS AND METHODS
Subjects
The current study is a retrospective cross-sectional study of 24,392 subjects; 602 suffering from severe or profound UHL (>70 dB on the affected side) and 23,790 normal hearing controls (<20 dB hearing loss). All participants in the current study were 19-year-old males who underwent physical examination for conscription at the Military Manpower Administration (MMA) from February 2015 to December 2016. In Korea, before joining the mandatory military service, all subjects underwent physical and psychological status evaluations by specialists from all departments of the MMA. The psychological status of the subjects was evaluated by the Korean Military Multiphasic Personality Inventory (KMPI) during the routine examination [19-24].
All normal hearing subjects were interviewed by one physician and did not have any remarkable past medical history. The hearing thresholds of the UHL subjects (n=602) were determined by pure tone audiometry (PTA) and auditory brainstem response, and they all showed normal air conduction thresholds on the contralateral ear (<20 dB hearing loss). The degree of UHL was evaluated by recent (within 3 months) PTA average. PTA average was defined as the average hearing threshold at 500, 1,000, 2,000, and 4,000 Hz. All UHL subjects included in the current study had severe or profound UHL. Participants who had been previously diagnosed with any psychiatric disorders such as depression, anxiety disorder, or schizophrenia were excluded from the study. Also, to maintain the homogeneity of the study group, subjects with unilateral conductive hearing loss or a history of previous temporal bone trauma or otologic surgical intervention were excluded.
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of the Clinical Research Institute at Chungbuk National University Hospital (IRB No. 2019-01-008). Considering a retrospective review study design, written informed consent was waived.
Korean Military Multiphasic Personality Inventory
The KMPI has been developed for use in Korean conscription and has the same scoring system in psychological evaluation as the MMPI [19-24]. As an objective psychological evaluation of conscription, KMPI is used to distinguish between psychologically healthy and unhealthy subjects, as well as to provide early screening for psychiatric disorders and criminal behavior [25].
The KMPI is composed of subscales such as the validity scales (faking-good, faking-bad, and infrequency), neurosis scales (anxiety, depression, and somatization), psychopathy scales (schizophrenia, personality disorder, and paranoia), and specific content scales (criminal and military-related scales). The KMPI produces scores for each scale, which are transformed into a standardized T-score [22]. Therefore, by comparing the T-scores of given groups, trends with regard to personality and psychopathology can be objectively analyzed same as in the MMPI. In this study, we evaluated the association between UHL and neurosis scale (anxiety, depression, and somatization), and psychopathy scale (schizophrenia, personality disorder, and paranoia).
Statistical analysis
Evaluation of the statistical significance of differences between groups was carried out using Student t-test. Statistical significance was set at P<0.05. The statistical comparisons were performed using IBM SPSS ver. 20.0 (IBM Corp., Armonk, NY, USA).
RESULTS
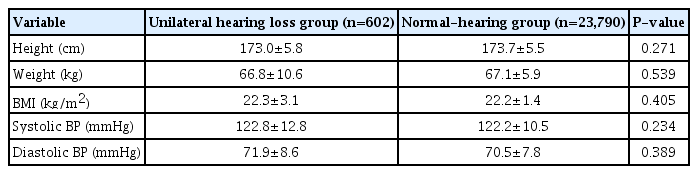
All 24,392 participants were 19-year-old Korean males without a history of previous psychiatric problems. Significant differences in baseline characteristics were not found between the UHL and normal-hearing groups. Table 1 summarizes the detailed baseline demographic data of the participants.
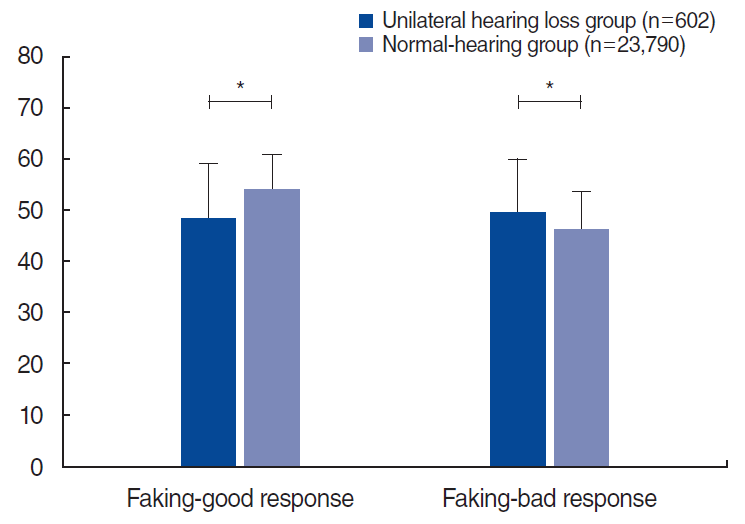
The results of the KMPI in the UHL group significantly differed from those in the normal-hearing group. In the validity scales, the UHL group showed a significantly lower faking-good response than that of the normal hearing group (48.3±10.7 vs. 54.1±6.8, P<0.001), and the faking-bad response of the UHL group was significantly higher than that of the normal-hearing group (49.6± 10.3 vs. 46.0±7.6, P<0.001) (Fig. 1). In the neurosis scales, all three subscales (anxiety, depression, and somatization) had markedly higher scores in the UHL group than in the normal hearing group (50.9±10.8 vs. 44.9±6.0 for the anxiety subscale; 51.0± 10.5 vs. 44.9±5.2 for the depression subscale; 51.1±10.4 vs. 45.1±6.81 for the somatization subscale, all P<0.001) (Fig. 2). Likewise, in the psychopathy scales, all three subscales (schizophrenia, personality disorder, and paranoia) had significantly higher scores in the UHL group (49.3±9.4 vs. 46.3±5.7 for the schizophrenia subscale; 51.1±11.2 vs. 44.3±5.8 for the personality disorder subscale; 51.1±10.5 vs. 45.7±3.7 for the paranoia subscale, all P<0.001) (Fig. 3).
DISCUSSION
UHL is a relatively common disease. Single-sided deafness, the most extreme form of UHL, has been reported to have a prevalence between 0.1% to 3.0% in the pediatric population [26]. However, despite its relatively high prevalence, UHL has been under-recognized as a problem and thus not properly managed in children and young adolescents [27]. Rather, UHL has been regarded as “a happy feature of a misfortune” because affected children show generally normal development [6]. The treatment options for UHL range from no intervention to FM amplification systems, conventional hearing aids, hearing aids with contralateral routing of signals, bone-anchored hearing aids, and cochlear implantation [28-31]. However, in real-world clinical situations, the majority of young people with UHL only receive preferential seating at the front of the class and are educated on the importance of protecting the contralateral ear with normal hearing.
Many studies have explored correlations between mental health disorders and bilateral hearing impairment in pediatric and young adolescent populations [32]. Most of these studies reported that hearing-impaired children exhibited poorer psychological and quality-of-life outcomes than their normal-hearing peers [33]. Fellinger et al. [32] reported that the lifetime rate of depression among children with bilateral hearing impairment was 26.3%, compared to 3.4% in their normal-hearing peers. However, not many studies have examined UHL. Since the 1970s, several investigators have demonstrated that children with UHL make more errors on sound localization tests due to the interaural time and intensity disparities [26,34]. Other reports indicated that adults with early-onset UHL showed problems in psychoacoustic performance such as sound localization and speech recognition in noisy environments [35-37]. However, to our knowledge, no studies have investigated the relationships between psychological problems and UHL. The authors hypothesized that UHL may be associated with psychological problems similar to those seen in bilateral hearing loss, and the results of the current study indicated that the UHL group showed a higher risk of neuropsychiatric disorders on various KMPI scales than the normal-hearing group.
In this study, we used three scales of the KMPI. First, the validity scales are composed of faking-bad response and fakinggood response. The reasons for faking bad can include making a “plea for help” and having a catastrophizing style, while the reasons for faking good include seeking to deny problems and desiring to appear psychologically healthy [38]. In the current study, the UHL group showed significantly higher faking-bad responses and lower faking-good responses than the normal-hearing group. In other words, the participants with UHL tended to exaggerate their symptoms more than their counterparts with normal hearing. Second, the neurosis scales are composed of anxiety, depression, and somatization subscales, which reveal the respondent’s tendency toward these neurotic statuses. Third, the psychopathy scales comprise the schizophrenia, personality disorder, and paranoia subscales, which also address the status of social relations. In this study, the UHL group showed statistically significantly higher scores on the neurosis and psychopathy scales than the normal-hearing group. These findings suggest that UHL may significantly impact the psychological state of affected individuals.
Based on the results of the current study because many children are at a higher risk of psychological problems related to their UHL, it appears necessary for us to begin to reassess our general approach to this population. The long-standing practice of simply recommending preferential classroom seating can no longer be considered the standard management approach. Parents, teachers, and physicians need to be well informed of affected children’s psychological status and progress. It is important to provide psychological support to school-aged subjects with UHL and advice to parents regarding their child’s psychological status. In addition, early detection of the child’s problems, clear delineation of psychological problems, and appropriate support can prevent frustration and secondary emotional reactions [28]. Moreover, a multidisciplinary approach for the initial assessment and active therapeutic interventions are needed in the outpatient clinic. Of course, one cannot overemphasize the importance of monitoring the good ear [39].
Although this is the first study to investigate the relationships between UHL and psychological problems in a large sample of subjects, this study has some limitations. First, the subjects analyzed in the current study are not representative of all subjects with UHL in the community. Only 19-year-old males were included in this study because the data were sourced from military conscription examinations. In other studies, it has been reported that depressive tendencies tended to decrease with age, and that being male was associated with lower anxiety and depression scores [40,41]. Second, the KMPI has not been widely documented or analyzed in the literature because its purpose is for a unique environment—that is, Korean military conscription. However, the KMPI has been officially approved for use by the Korean government and is highly similar to the MMPI as a tool for psychological evaluation, to the point that it even uses the same scoring system [25,42]. In addition, when interpreting KMPI results, the between-group differences in each subscale cannot be considered clinically significant. Therefore, the results of the current study should not be interpreted as a hasty generalization; instead, they should be considered as reflecting the possibility that UHL may have psychopathological effects.
In conclusion, 19-year-old males with UHL tended to have more abnormal results on personality testing than controls with normal hearing, suggesting that UHL may be related to a higher risk of psychopathology. It is hoped that the findings of this study will prompt our colleague otolaryngologists and audiologists to pay more attention to this population, and we suggest that clinicians should evaluate the psychopathological aspects of patients with UHL.
HIGHLIGHTS
▪ Few studies have investigated psychopathological abnormalities in individuals with unilateral hearing loss.
▪ Nineteen-year-old males with unilateral hearing loss tended to have more abnormal results on personality tests than controls with normal hearing.
▪ Unilateral hearing loss may be related to a higher risk of psychopathology.
Notes
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conceptualization: JJS, HJJ. Data curation: SK, EK, YSC. Formal analysis: SK, EK, YSC. Investigation: SK, EK, YSC. Methodology: JJS, EK, HJJ. Writing–original draft: JJS, EK, HJJ. Writing–review & editing: JJS, EJK, HJJ.