Development of a Serum-Free Culture Method for Endothelial Cells of the Stria Vascularis and Their Pro-inflammatory Secretome Changes Induced by Oxidative Stress
Article information
Abstract
Objectives
Reactive oxygen species in the stria vascularis (SV) of the cochlea may be involved in the pathogenesis of sensorineural hearing loss. However, the effects of oxidative stress on SV endothelial cells (SV-ECs) remain largely unknown, and no feasible in vitro cell culture model exists for the functional study of SV-ECs.
Methods
We isolated primary SV-ECs from the SV of neonatal mice. The apoptosis-reducing effects of fibronectin in SV-ECs cultured with serum-free medium were determined using β-galactosidase staining and flow cytometry. SV-ECs incubated in serum-free medium were treated with various H2O2 concentrations to evaluate the effects of H2O2 on their viability. The secretome of SV-ECs treated with or without H2O2 (100 μM or 500 μM) was analyzed using high-resolution mass spectrometry. The function of the SV-EC secretome was evaluated by a macrophage assay.
Results
We successfully isolated and characterized the SV-ECs. Treatment with H2O2 at concentrations up to 500 μM for 2 hours and further incubation with serum-free medium in plates precoated with fibronectin showed no significant effect on apoptosis. Compared to the control SV-ECs, the amount of differential proteins in the secretome of SV-ECs stimulated with 500 μM H2O2 was much higher than in those treated with 100 μM H2O2. Kyoto Encyclopedia of Genes and Genomes and Gene Ontology analyses suggested that the proteins differentially expressed in SV-ECs treated with 500 μM H2O2 were involved in the regulation of multiple signaling pathways and cellular processes. The secretome of H2O2-stimulated SV-ECs exhibited significant pro-inflammatory effects on macrophages.
Conclusion
We successfully established an in vitro serum-free culture method, identified the differential proteins released by oxidative stress-induced ECs and their functions, and revealed the pro-inflammatory effects of the secretome of H2O2-stimulated SV-ECs. Therefore, SV-ECs might elicit immunoregulatory effects on bystander cells in the microenvironment of oxidative stress-induced cochlea, especially cochlear macrophages.
INTRODUCTION
Hearing loss (HL), defined as reduced hearing sensitivity above a hearing threshold of 20 dB, may affect speech and language development in children and cause social and occupational problems in adults. It has been reported that nearly 2.5 billion people are projected to develop HL by 2050, which will extensively reduce their quality of life and causes substantial economic losses to society [1]. In particular, sensorineural hearing loss (SNHL), which accounts for about 90% of all HL, is the most common form of hearing impairment. However, the mechanisms involved in the pathogenesis of SNHL remain to be further elucidated.
The blood-labyrinth barrier (BLB), located in the intermediate of the stria vascularis (SV), is critical for the maintenance of the unique ionic composition of endolymph, which is responsible for the mechanical transduction of auditory signals in cochlear sensory hair cells. The BLB consists of the basement membrane, endothelial cells (ECs), pericyte cells (PCs) and perivascular resident macrophage-like melanocytes (pVM/Ms) [2], and it plays an important role in maintaining the barrier integrity of the BLB [3]. In particular, the ECs in the SV (SV-ECs), which lie along the entire microvasculature and limit the paracellular transport, are often considered to play a significant role in maintaining the balance of cochlear perilymph [4,5]. It has been widely reported that after stimulation by factors such as hypoxia and oxidative stress, vascular ECs from organs such as the lung [6], heart [7] and kidney [8] were able to release numerous proinflammatory components involved in the regulation of the bystander cells and contributed to the pathogenesis of diseases, in addition to the destruction of endothelial permeability [9]. It has also been demonstrated that ECs secrete exosomes carrying different proteins and microRNAs (miRNAs) in response to different types of cellular stress, indicating that ECs could exhibit exosome-mediated cell communication [10]. Based on this evidence, we hypothesized that the SV-ECs might also elicit regulatory effects on the cochlear bystander cells in response to SNHL-related risk factors, such as noise exposure. Additionally, a method for isolating and culturing of SV-ECs has been previously reported by Neng et al. [11], but the culture method in this protocol required the addition of fetal bovine serum (FBS). To reveal the secretome changes of SV-ECs after oxidative injury, it is essential to establish a serum-free in vitro culture method for SV-ECs to avoid contamination by serum-derived proteins.
It has been widely reported that oxidative stress in the cochlea is one of the most significant factors leading to hearing damage in noise-induced or aging-related SNHL, although the mechanisms remain to be further elucidated [12,13]. Exposure to SNHL-related risk factors triggers the production of reactive oxygen species in the cochlea, which finally leads to cellular damage as well as apoptotic or necrotic cell death [14,15]. Previous studies have demonstrated that both necrosis and apoptosis commonly occur in the sensory epithelium after exposure to risk factors such as noise, resulting in temporary or permanent damage to hair cells, especially the outer hair cells. Although sensory hair cells are usually considered to be the main targets of SNHL-related risk factors, recent studies have also found significant changes in the ligament and SV of the cochlea [16,17]. It has been demonstrated that exposure to SNHL-related risk factors could induce early cellular apoptotic signals in SV-ECs [18], aggravate the production of reactive oxygen species, and promote cellular injury in marginal cells of the inner ear. However, the role of oxidative stress in the dysfunction of SV-ECs has never been reported.
In this study, we sought to establish a serum-free culture method for SV-ECs in vitro and explore the effects of oxidative stress on apoptosis in SV-ECs. We also revealed the changes in the secretome of SV-ECs induced by oxidative stress and analyzed the function of these differential proteins, thereby providing a novel in vitro model for the study of SV-ECs that contributes to our understanding of the role of oxidative stress in the SV after exposure to SNHL-related factors.
MATERIALS AND METHODS
Animals
Wild-type neonatal mice at postnatal day 10–15 were purchased from Laboratory Animal Center, Sun Yat-sen University. The procedures performed on animals were approved by the Institutional Animal Care and Use Committee of The First Affiliated Hospital, Sun Yat-sen University in compliance with the National Institutes of Health guidelines for the care and use of laboratory animals.
Isolation and selective culture of SV-ECs
The isolation and culture of ECs were performed as previously reported [11]. The mice were decapitated after being euthanized by carbon dioxide asphyxiation and the heads were placed in 75% ethanol. The cochlea were rapidly removed and placed in a Petri dish with cold, fresh Hanks’ balanced salt solution. The stria vascularis was isolated from cochlea using sterile procedures in ice-cold Hanks’ balanced salt solution, placed intactly on poly-L-lysin-coated 35 mm culture dishes with ECs culture medium (ECM basic medium+10% FBS+1% Endothelial Cell Growth Supplement+1% Penicillin-Streptomycin), and teared into 0.1 mm–0.2 mm3 with tweezers. The fragmented tissues were attached to the Petri dish, and further incubated at 37 °C in 5% CO2 with ECs culture medium.
Flow cytometry analysis
For isolation of SV-ECs, the whole cells isolated from stria vascularis tissues were collected for flow cytometry cell sorting after being selectively cultured in EC medium as previously reported. Briefly, the cells were incubated with 20 μg/mL fluorescein isothiocyanate (FITC)-conjugated isolectin B4 (BSI-B4; Sigma) at 4 °C for 30 minutes, and then immediately analyzed and sorted with a BD Influx Cell Sorter (Becton; Dickinson and Company). For characterization of peritoneal macrophages (Mφ), cells were stained with anti-F4/80 (eBioscience) and antiCD11b (BioLegend) and finally analyzed on a CytoFLEX flow cytometer (Beckman Coulter).
Culture and treatment of SV-ECs
Primary ECs were incubated with SV-ECs culture medium and passaged to P4 at the ratio of 1:3. To evaluate the dose-dependent effects of H2O2 on SV-ECs, the cells were pre-treated with H2O2 at 100 μM, 200 μM, 500 μM and 1,000 μM for 2 hours in EC medium. The treatment of H2O2 was followed by another 24-hour incubation in FBS-free EC medium in cell culture plates (CCPs) precoated with (F-CCP) or without (non-F-CCP) 30 μg/mL fibronectin (F; Corning) as indicated in some experiments. For proteomic analyses SV-ECs secretome, the SV-ECs at P4 were incubated with 20 mL ECs medium in fibronectin-pretreated 150 cm2 cell culture dishes, pretreated with 100 μM or 500 μM H2O2 for 2 hours, and further incubated with FBS-free ECs medium for 24 hours after 3 washes with PBS.
Immunofluorescence
SV-ECs were fixed for 30 minutes at room temperature with 4% paraformaldehyde in PBS, and then incubated with 0.3% Triton X-100 and 10% donkey-serum in PBS for 30 minutes for permeabilization and blocking, respectively. Subsequently, SV-ECs were incubated with antibody to von Willebrand factor (Abcam). After overnight incubation, the SV-ECs were washed with PBS and incubated with secondary antibodies and Alexa Fluor 488-conjugated Phalloidin (Thermo Fisher Scientific) for 1 hour at room temperature. The nuclei were stained with DAPI (4´,6-diamidino-2-phenylindole; Roche). The SV-ECs were finally photographed using a Leica DM4B microscope (Leica).
Polymerase chain reaction
Total RNA was extracted using TRIzol (Invitrogen) according to the manufacturer’s protocol. Complementary DNA (cDNA) was synthesized using the PrimeScript II 1st strand cDNA synthesis kit (Takara). The products of the DNA of von Willebrand factor (vWF) in SV-ECs were identified by DNA electrophoresis on 1.5% agarose gel. Quantitative real time-polymerase chain reaction (PCR) was performed using the SYBR Green Pro Taq HS qPCR kit (AG). The data were calculated by 2−ΔΔCt method and levels messenger RNA (mRNA) were normalized to β-actin. PCR primers used in our study were as follows:
vWF: 5´-TGTTCATCAAATGGTGGGCAGC-3´ (forward), 5´-ACAGACGCCATCTCCAGATTCA-3´ (reverse); IL-6: 5´-TGTTCATCAAATGGTGGGCAGC-3´ (forward), 5´-ACAGACGCCATCTCCAGATTCA-3´ (reverse); TNF-α: 5´-ATGAGCACAGAAAGCATGA-3´ (forward), 5´-AGTAGACAGAAGAGCGTGGT-3´ (reverse); IL-1β: 5´-AACCTGCTGGTGTGTGACGTTC-3´ (forward), 5´-CAGCACGAGGCTTTTTTGTTGT-3´ (reverse); Arg1: 5´-ATGAAGAGCTGGCTGGTGTG-3´ (forward), 5´-GCCAGAGATGCTTCCAACTG-3´ (reverse); Fizz1: 5´-CCCTCCACTGTAACGAAGACTC-3´ (forward); 5´-CACACCCAGTAGCAGTCATCC-3´ (reverse); β-actin: 5´-GAGGTATCCTGACCCTGAAGTA-3´ (forward), 5´-CACACGCAGCTCATTGTAGA-3´ (reverse).
Apoptosis analysis
SV-ECs were seeded at a density of 5×104 per well in 24-well plates with SV-ECs medium or FBS-free SV-ECs medium as indicated. In some experiments, the SV-ECs were treated with different concentration of H2O2 as indicated. The supernatants were collected for staining using the Annexin V-FITC/PI apoptosis detection kit (Elabscience) according to the manufacturer’s instructions. The ECs were finally analyzed on a CytoFLEX Flow Cytometer (Beckman Coulter) and analyzed using CytExpert software (Beckman Coulter).
β-galactosidase staining
To evaluate the effect of oxidative stress on senescence of SVECs, β-galactosidase staining was performed. Briefly, SV-ECs were seeded at a density of 5×104 per well in 24-well plates with SV-ECs medium or FBS-free SV-ECs medium, stimulated by different H2O2 concentrations as indicated, and collected for β-galactosidase staining according to the manufacturer’s instructions. The images were photographed using an microscope (Olympus).
Cell viability assay
Cells were seeded at a density of 1×104 per well in 96-well plates and cultured similarly to the apoptosis analysis experiments. Cell Counting Kit-8 (Yeasen) colorimetric assay was used to determine cell viability according to the manufacturer’s protocol. The absorbance was read at 450 nm with a BioTek Gen 5 microplate reader (BioTek).
High resolution mass spectrometry
The ECs were cultured and treated with 100 μM or 500 μM H2O2 as mentioned above. The supernatant was collected and centrifuged at 2,000 g for 5 minutes to remove cell debris. Subsequently, the supernatants were centrifuged at 5,000 g for 4–5 hours at 4 °C using a 3 kDa MWCO ultrafiltration tube (Thermo Fisher Scientific) for concentration. The protein was extracted from the concentrated ECs supernatants and quantitative analysis was performed using the high-resolution mass spectrometer, which were technically supported by BGI Co., Ltd. Briefly, quality control of protein extraction was performed by Bradford protein quantification and SDS-PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis) electrophoresis. Freeze-dried the peptide liquid was obtained from 100 μg protein after proteolysis, which were further reconstituted with mobile phase A (2% ACN, 0.1% FA), centrifuged and separated by a Shimadzu LC20AD model nanoliter liquid chromatograph. Subsequently, the separated peptides were passed to an ESI tandem mass spectrometer and data were acquired after the mass spectrometer parameters were set. The differential proteins in each comparison group were calculated and the significance test was performed using Welch’s t-test. Significant differences were determined by setting the multiple of difference >1.5 and P<0.01 as the criteria. The enrichment analysis of the differential proteins was performed accordingly.
Isolation and culture of primary peritoneal Mφ
Peritoneal Mφ were harvested by peritoneal lavage with 5 mL of cold 2 mM EDTA and plated in Dulbecco’s modified Eagle medium supplemented with 10% FBS and penicillin/streptomycin. The Mφ were seeded at the density of 5×105/well in 24-well plates, stimulated with 5 μg/mL or 50 μg/mL of concentrated SV-ECs supernatants for 6 hours and finally collected for PCR. The state of the peritoneal Mφ is M0.
Enzyme linked immunosorbent assay
Levels of interleukin (IL)-6 in the supernatant was quantified by enzyme linked immunosorbent assay (ELISA) using ELISA kits (NeoBioscience Technology). Absorbance at 450 nm was read on a BioTek Gen 5 microplate reader (BioTek).
Gene Ontology and Kyoto Encyclopedia of Genes and Genomes analyses
Gene Ontology (GO) enrichment analysis (http://www.geneontology.org/) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis (http://www.genome.jp/kegg/pathway.html) were used to illuminate the biological functions and pathways by following the instructions.
Statistical analyses
Statistical analyses were performed using Prism software (GraphPad Software). All values were expressed as mean±standard error of the mean for each group. Statistical significance was assessed by using independent two-tailed Student t-test for single comparisons or one-way analysis of variance with Tukey’s correction for multiple comparisons as indicated. A P-value less than 0.05 was considered statistically significant.
RESULTS
Establishing a serum-free culture method for SV-ECs
To obtain sufficient primary SV-ECs for functional analyses, we first isolated SVs from the cochleae of neonatal mice. All SV cells were selectively cultured in EC growth medium and the SV-ECs were sorted by flow cytometry analysis (Fig. 1A), as previously reported [11]. The isolated SV-ECs were characterized by morphology with bright field microscopy (Fig. 1B and C) and the expression of vWF as determined by flow cytometry analysis (Fig. 1D), immunofluorescence (Fig. 1E) and PCR gene analysis (Fig. 1F), respectively. Our data suggested that SV-ECs were successfully isolated from SV.
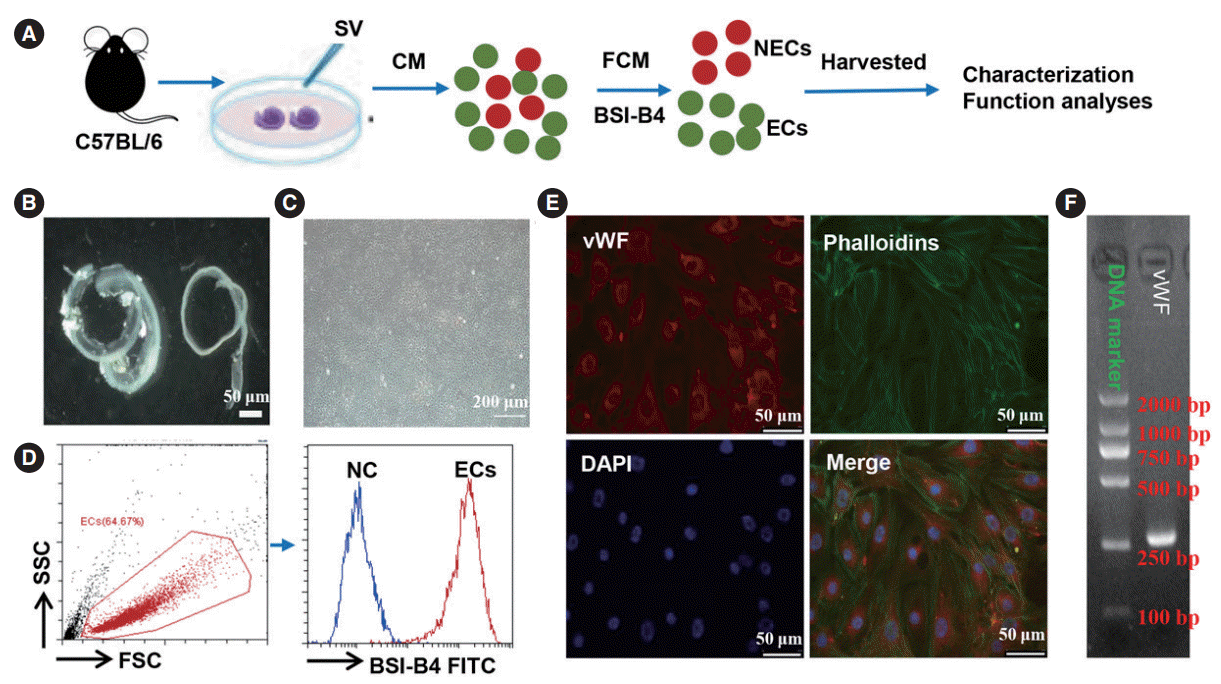
Isolation and characterization of stria vascularis endothelial cells (SV-ECs). (A) Schematic diagram illustrating the procedure of isolating ECs. (B, C) The cochlear lateral wall was fully isolated from the cochlea and placed in fresh ice-cold perilymph solution (B), and cochlear ECs were selectively cultured in EC growth medium (C). (D) ECs were characterized by flow cytometry analysis for cell purity. (E) Immunofluorescent images of ECs probed for phalloidins (green), von Willebrand factor (vWF; red), and nuclei (blue). (F) Polymerase chain reaction gene analysis of an EC marker (vWF). CM, conditioned medium; FCM, flow cytometry; NEC, non-endothelial cell; SSC, side scatter; FSC, forward scatter; FITC, fluorescein isothiocyanate; BSI-B4, isolectin B4; DAPI, 4´,6-diamidino-2-phenylindole.
Since we aimed to analyze the oxidative stress-induced secretome changes in SV-ECs, the presence of FBS in the supernatant would significantly interfere with the analysis of the ECs secretome. Thus, we next explored serum-free culture conditions for SV-ECs to avoid contamination with serum-derived proteins. It has been previously reported that bovine pituitary extract (BPE) [19], a supplement for serum-free culture enriched in growth factors that highly promote mitotic activity, as well as fibronectin [20], a plasma protein that exhibits structural and adhesive properties in cell-associated fibrillar matrices, were able to significantly reduce apoptosis in vascular ECs [19,20]. Therefore, we further explored whether the addition of BPE and fibronectin to the EC culture system would significantly reduce apoptosis in SV-ECs. The SV-ECs were cultured with different concentrations of BPE in F-CCP or non-F-CCP for 24 hours. We found that the SV-ECs incubated in F-CCP showed similar morphologies to those incubated with the EC medium, regardless of the concentration of BPE (Fig. 2A). However, we observed significant numbers of floating and apoptotic cells among the SV-ECs incubated with different concentrations of BPE in non-F-CCP (Fig. 2A). Similarly, we also noted that the proportion of apoptotic cells was significantly reduced in SV-ECs cultured in F-CCP, but not those with the addition of BPE to the medium, as demonstrated by flow cytometry analysis (Fig. 2B and C). Taken together, our data suggested that fibronectin precoating in the plate for SV-ECs culture was able to maintain the vitality of ECs in an FBS-depleted medium.
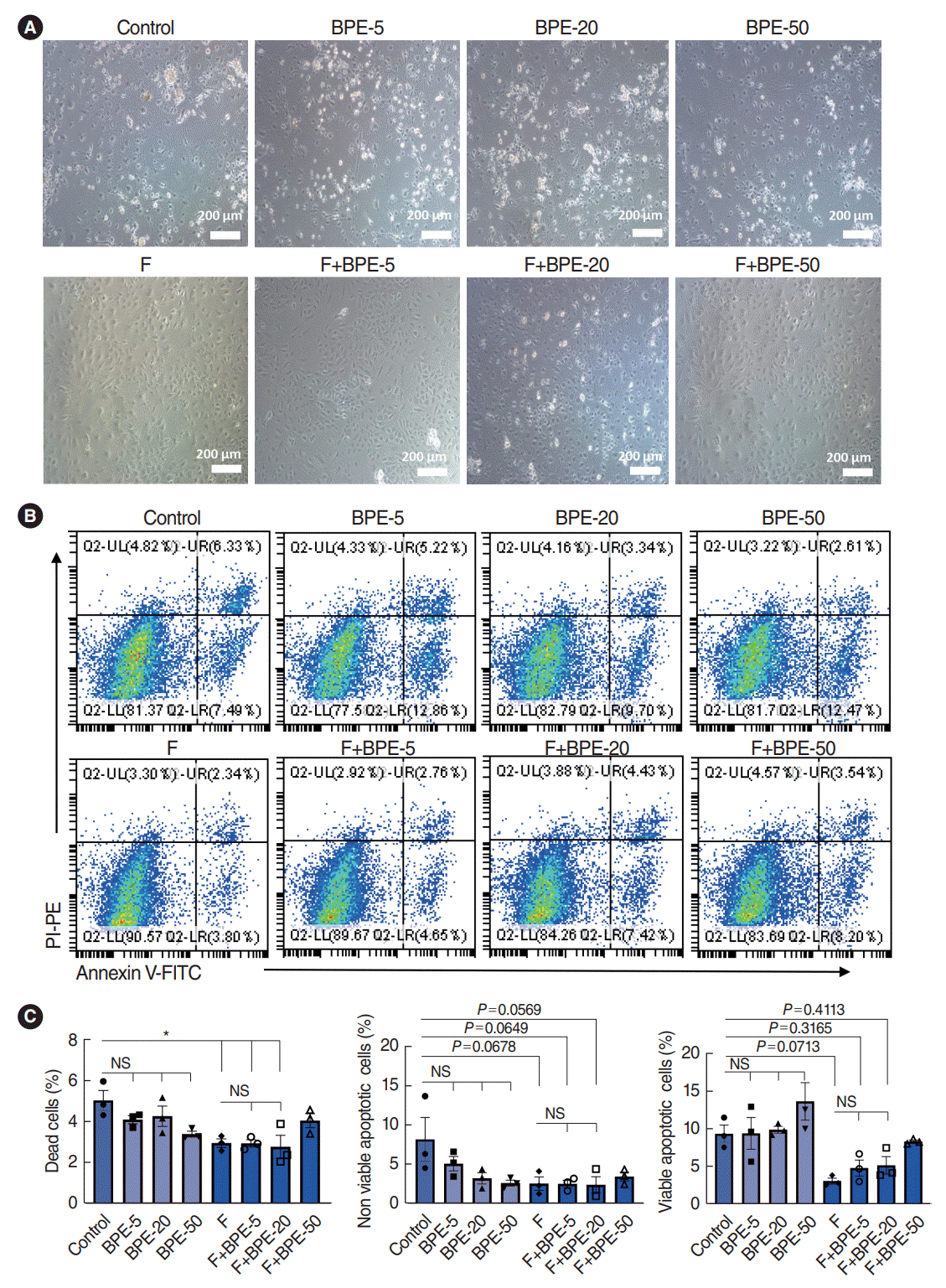
Fibronectin maintained the viability of stria vascularis endothelial cells (SV-ECs) in serum-free culture medium. (A) Fibronectin and bovine pituitary extract (BPE) were selected to explore the optimum conditions for serum-free culture of SV-ECs. SV-ECs were treated with different concentrations of BPE in serum-free culture medium for 24 hours in the presence (upper) or absence (lower) of fibronectin. Bright-field microscopy showed that the SV-ECs were in a good condition in the serum-free culture medium with fibronectin-precoated plates, and BPE showed no significant effects on reducing the apoptosis of SV-ECs in the serum-free medium. (B, C) Flow cytometry analyses suggested that the apoptosis rate of SV-ECs in the serum-free culture medium was significantly reduced among SV-ECs cultured in fibronectin-precoated plates. F, fibronectin; UL, upper left; UR, upper right; LL, lower left; LR, lower right; FITC, fluorescein Isothiocyanate; PI, propidium iodide; PE, P-phycoerythrin; NS, not significant. *P<0.05 according to one-way analysis of variance for multiple comparisons.
Fibronectin reduced the death rate of ECs induced by H2O2 in vitro
It’s been well demonstrated that oxidative stress is one of the key factors that contributes to the pathogenesis of SNHL; thus, we further explored the effects of H2O2 on the viability of SV-ECs. First, the SV-ECs incubated with EC medium were stimulated with various concentrations of H2O2 for 2 hours. Bright-field microscopy suggested that the SV-ECs stimulated with 100 μM, 200 μM, and 500 μM H2O2 maintained similar morphology to the control SV-ECs, and the SV-ECs started to detach from the culture plate after being stimulated with 1,000 μM H2O2. Similarly, the percentages of senescent cells for the SV-ECs stimulated with 100 μM, 200 μM, and 500 μM H2O2 were similar to the control cells, as demonstrated by β-galactosidase staining (Fig. 3A). In addition, flow cytometry analysis suggested that the percentages of apoptotic cells were only significantly increased in SV-ECs stimulated with 1,000 μM H2O2, but not in those with lower concentrations of H2O2 (Fig. 3B and C).
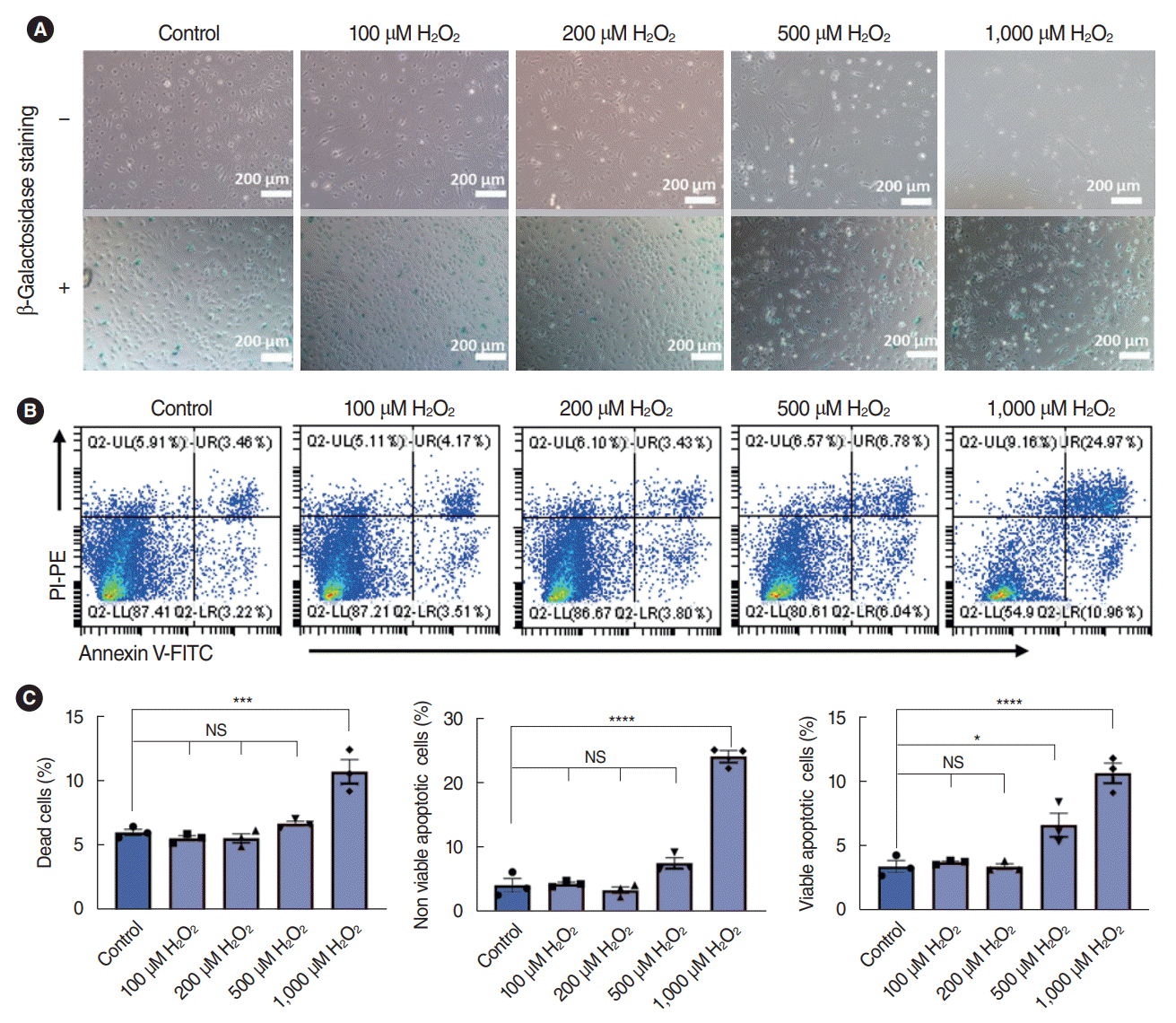
Dose-dependent effects of H2O2 on stria vascularis endothelial cells (SV-ECs) cultured with complete culture medium (CCM). (A) Bright-field microscopy with (lower) or without (upper) β-galactosidase staining for ECs pretreated with various concentrations of H2O2 for 2 hours in CCM. The SV-ECs were stimulated with 100 μM, 200 μM, 500 μM, and 1,000 μM H2O2 for 2 hours, and SV-ECs started to detach from the plate (upper) and become significantly senescent (lower) in response to stimulation by 1,000 μM H2O2 compared to the control cells. (B, C) Apoptotic analysis for ECs pretreated with various concentrations of H2O2 for 2 hours in CCM. Values are presented as mean±standard error of the mean (n=3). PI, propidium iodide; PE, P-phycoerythrin; FITC, fluorescein Isothiocyanate; FITC, fluorescein Isothiocyanate; UL, upper left; UR, upper right; LL, lower left; LR, lower right; NS, not significant. *P<0.05, ***P<0.001, ****P<0.0001 according to one-way analysis of variance.
Furthermore, we investigated whether the SV-ECs stimulated with H2O2 were able to maintain their viability in serum-free medium. The SV-ECs were stimulated with H2O2 for 2 hours in EC medium and then further incubated with serum-free medium for 24 hours in a fibronectin-coated CCP. We found that the cell viability of SV-ECs stimulated with 100 μM, 200 μM, and 500 μM H2O2 was similar to that of control SV-ECs, while those treated with 1,000 μM H2O2 showed significantly reduced viability (Fig. 4A). Additionally, β-galactosidase staining demonstrated that H2O2-stimulated SV-ECs incubated with serum-free medium showed significantly fewer senescent cells in F-CCP than in non-F-CCP (Fig. 4B and C), suggesting that precoating with fibronectin was required to reduce apoptosis in the H2O2-induced ECs.

Dose-dependent effects of H2O2 on stria vascularis endothelial cells (SV-ECs) cultured in serum-free medium. (A) Cell viability assay for ECs pretreated with various concentrations of H2O2 for 2 hours, followed by incubation with serum-free medium for 24 hours in fibronectin-precoated cell culture plates. (B, C) Bright-field microscopy for β-galactosidase staining of ECs pretreated with 100 μM or 500 μM H2O2 for 2 hours, followed by incubation with serum-free medium for 24 hours in cell culture plates with or without precoating of fibronectin. Values are presented as mean±standard error of the mean (n=3). CCM, complete culture medium; SFM, serum-free culture medium; NS, not significant. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 according to one-way analysis of variance.
Overall, our data suggested that ECs stimulated with H2O2 at concentrations lower than 500 μM for 2 hours and further incubated in serum-free medium for 24 hours in F-CCP were able to maintain their viability.
Secretome changes of SV-ECs induced by oxidative stress
As described above, we successfully established a method for incubating SV-ECs with serum-free medium, which enabled us to investigate changes in the secretome and the related function. The secretome released by SV-ECs stimulated with 100 μM (H2O2-100 μM) and 500 μM H2O2 (H2O2-500 μM) or PBS (control) was investigated by a proteomics analysis. In total, we identified 610, 634, and 897 proteins in the secretome derived from control SV-ECs and SV-ECs treated with 100 μM and 500 μM H2O2, respectively. To increase the confidence levels of the analyses, we defined differential proteins with the criteria of a multiple of difference >1.5 and a P<0.01. We found that the level of differential proteins between the control SV-ECs and the SV-ECs treated with 100 μM H2O2 was very low, while the SV-ECs treated with 500 μM H2O2 showed a higher level of differential proteins in comparison to the control SV-ECs and SV-ECs treated with 100 μM H2O2 (Fig. 5A-C). The 48 differential proteins in ECs with or without treatment with 500 μM H2O2 were mainly involved in glycolysis, carbon metabolism, biosynthesis of amino acids, and the HIF-1 signaling pathway according to the KEGG analysis (Fig. 5D). In addition, the GO analysis suggested that the differential proteins, which are important components of different parts of cells, are significantly involved in cellular processes, metabolic processes, and biological regulation in terms of biological processes, and highly related to binding, catalytic activity, and structural molecule activity in terms of molecular function (Fig. 5E).
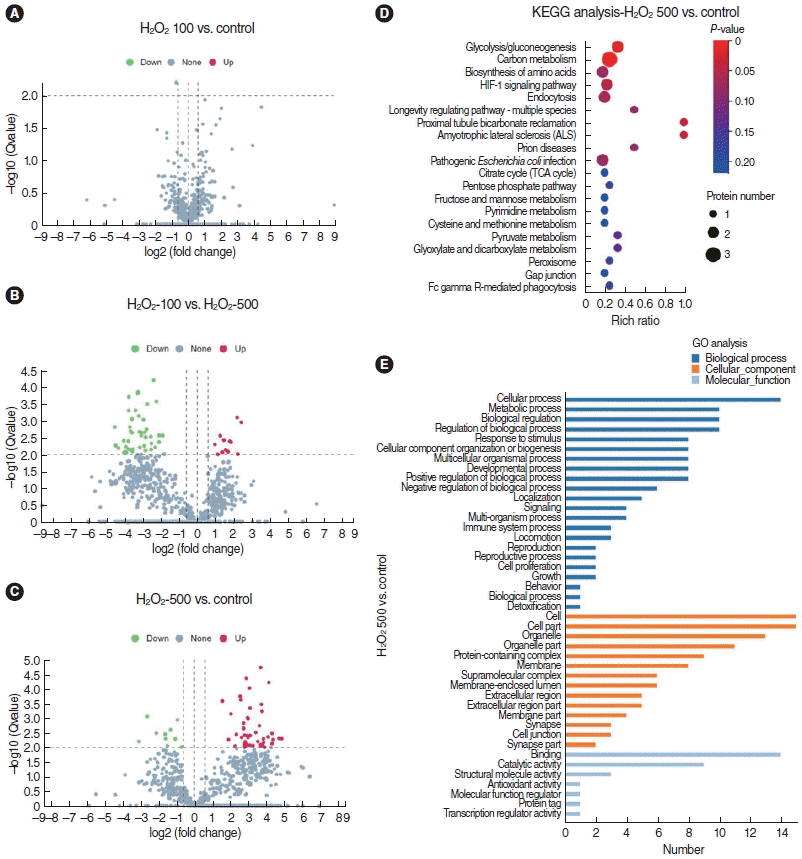
LC-MS/MS analysis of supernatant of stria vascularis endothelial cells (SV-ECs) with H2O2. (A-C) Volcano plots for the differential expression analysis of the secretome released by ECs treated with or without H2O2 as indicated. (D, E) Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis (D) and Gene Ontology (GO) analysis (E) showed the function of the differential secretome between control cells and ECs treated with 500 μM H2O2.
Oxidative-stress induced SV-ECs might secrete certain functional proteins to regulate the function of bystander cells in the cochlea exposed to SNHL-related risk factors, such as hair cells and Mφ. Therefore, the differential proteins we identified in the secretome of ECs treated with or without H2O2 might play an important role in the pathogenesis of SNHL. However, much additional work will be required to identify the key proteins that potentially mediate hearing injury after noise exposure and the detailed mechanisms.
Effect of the SV-ECs’ secretome on Mφ
To evaluate the effect of the secretomes of SV-ECs on Mφ, we harvested peritoneal Mφ (Fig. 6A), and flow cytometry analysis showed that the purity of Mφ is about 95% (Fig. 6B). The Mφ were stimulated with PBS, the H2O2-100 μM secretome, or the H2O2-500 μM SV-EC secretome for 6 hours. Compared with the control, the levels of proinflammatory cytokines, such as IL-6, TNF-α, and IL-1β were significantly increased by the H2O2-100 μM and H2O2-500 μM SV-EC secretomes in a dose-dependent manner, as demonstrated by PCR (Fig. 6C). Similarly, we also found that the level of IL-6 in the supernatants was significantly higher in Mφ stimulated with H2O2-100 μM and H2O2-500 μM SV-EC secretome (Fig. 6D). Consistently, both the H2O2-100 μM and H2O2-500 μM SV-EC secretomes elicited no effect on the levels of anti-inflammatory cytokines such as Arg1 and Fizz1 (Fig. 6E). In total, our data demonstrated that the secretome released by SV-ECs stimulated with H2O2 exhibited pro-inflammatory effects on Mφ.

Effects of the secretome of stria vascularis endothelial cells (SV-ECs) stimulated with or without H2O2 on peritoneal macrophages (Mφ). (A) Bright-field microscopy of Mφ harvested from the peritoneal cavity. (B) Peritoneal Mφ were characterized by flow cytometry analysis. (C, D) The levels of pro-inflammatory cytokines, including interleukin (IL)-6, tumor necrosis factor (TNF)-α and IL-1β, were increased in the secretomes of SV-ECs stimulated by H2O2 in a dose-dependent manner, as demonstrated by polymerase chain reaction (C) and enzyme-linked immunosorbent assay (ELISA) (D). (E) The secretomes of SV-ECs stimulated by H2O2 showed no effect on levels of anti-inflammatory cytokines, such as Arg1 and Fizz1. SSC, side scatter; FSC, forward scatter; NS, not significant. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 according to one-way analysis of variance.
DISCUSSION
In this study, for the first time, we established a serum-free in vitro culture method for SV-ECs. Based on this in vitro model, we further revealed the differential proteins secreted by ECs induced by oxidative stress and their potential function and demonstrated that the secretome of H2O2-stimulated SV-ECs elicited pro-inflammatory effects on Mφ.
The BLB is one of the most important parts of the SV. It consists of ECs, PCs, and pVM/Ms, and plays a key role in the maintenance of the ion composition of the endolymph and production of endocochlear potential in the scala media [3]. It has been previously reported that SV injury is one of the key factors responsible for the pathogenesis of SNHL related to noise or aging [18,21], but the related mechanisms remain to be further elucidated, especially the regulatory role of SV-ECs. Evidence supports that vascular ECs can also induce pathogenic tissue niches that mediate the pathogenesis of some diseases, such as inflammatory lung injury [6], coronary artery disease [7] and chronic kidney disease [8]. Therefore, we hypothesized that SV-ECs might also display similar regulatory effects on Mφ in certain types of SNHL. The first step for investigating the function of SV-ECs is to establish the isolation and serum-free culture method. In 2013, Neng et al. [11] developed a protocol for isolating the three cell types on a blood-labyrinth, which made it possible to investigate cell-cell interactions based on an in vitro cell-cultured model. In this study, we first successfully isolated ECs from the SV following the protocol of Neng et al. [11]. To study the secretome of the ECs, we further explored the serum-free culture method. Previous studies have demonstrated that the addition of either BPE [19] or fibronectin [20] to the culture system could significantly reduce the apoptosis of ECs, but this has never been reported for SV-ECs. Our data showed that, for the primary SV-ECs we isolated, precoating of fibronectin on the CCP, but not the addition of BPE to the medium, significantly increased the viability of SV-ECs. Therefore, the serum-free culture method for SV-ECs provides a cell-culture-based model to study the role of SV-EC dysfunction in SNHL.
It has been reported that noise stress induced reactive oxygen species in the lateral wall of the cochlea, which would further cause oxidative damage to the cochlear SV [18]. However, the responses of SV-ECs to oxidative stress remained unclear. We investigated the effects of various concentrations of H2O2 on the viability of ECs, and we found that the concentrations of H2O2 lower than 500 μM showed no significant effect on the viability of SV-ECs. The concentration is higher than the concentration of H2O2 that elicited apoptosis-promoting effects on other vascular ECs as previously reported [22]. This discrepancy could have occurred because we only stimulated the ECs for 2 hours to mimic short-time sudden noise exposure, which is shorter than that reported in the other study. We also demonstrated that the SV-ECs were able to further maintain their viability in serum-free medium after being stimulated with 500 μM H2O2 for 2 hours. To investigate the effects of oxidative stress on SV-ECs using this in vitro cell culture model, it is required to minimize apoptosis in SV-ECs to prevent contamination by apoptotic proteins. Therefore, our data demonstrated that we have successfully developed an in vitro model of oxidative stress-induced SV-ECs that would make it possible to investigate the potential role of oxidative stress-induced injury to SV-ECs in the development of HL.
In previous reports, SV-ECs, in cooperation with PCs and PVM/Ms, were mainly considered to function by maintaining the barrier integrity, which could be disrupted by oxidative stress induced by noise exposure. Nevertheless, it has never been reported whether changes in the ECs’ secretome would contribute to HL after oxidative stress. Our data, for the first time, revealed changes in the secretome in SV-ECs after oxidative stress-induced injury based on the in vitro cell culture method we established. We also investigated the potential function of the differential proteins released by ECs in response to H2O2. In particular, we demonstrated that the secretome released by SV-ECs stimulated with H2O2 exhibited significant pro-inflammatory effects on Mφ. Our data suggested that the differential proteins in the SV-EC secretome exhibited proinflammatory effects.
We acknowledge that there are some limitations of our study. The regulatory effects of the EC secretome in the cochlea niche and its role in the pathogenesis of SNHL were not explored in the present study. An animal study would also be important to confirm the role of the SV-EC secretome in oxidative stress-induced cochlear injury, especially its effects on cochlear Mφ. Therefore, further research is required to answer these questions.
In summary, we successfully established a serum-free culture method for ECs in the SV, which enabled us to further investigate changes in the secretome of ECs after oxidative stress and the function of the differential proteins. Our study provides a novel approach to and understanding of the pathogenesis of SNHL in terms of oxidative injury of SV-ECs.
HIGHLIGHTS
▪ We successfully developed an in vitro model of oxidative stress-induced stria vascularis endothelial cells (SV-ECs) using a serum-free culture method.
▪ The secretome of H2O2-stimulated SV-ECs showed pro-inflammatory effects on macrophages.
▪ Our in vitro serum-free culture method could contribute to understanding the role of oxidative stress in the SV.
Notes
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conceptualization: GXX, SBF. Data curation: GXX, SBF. Formal analysis: YY. Investigation: YY, XRW, HTC, WYH, LXF. Methodology: YY, XRW. Project administration: GXX, SBF. Resources: GXX, SBF. Software: YY. Supervision: GXX, SBF. Validation: GXX, SBF. Visualization: YY, XRW. Writing–original draft: YY, SBF. Writing–review & editing: GXX, SBF.
Acknowledgements
This study was supported by grants from the National Key Research and Development Program of China (grant no. 2020YFC 2005204), Science Plan Fund of Guangzhou (grant no. 202103 000079), NSFC (grant no. 82101185), Guangdong Basic and Applied Basic Research Foundation (grant no. 2020A1515110794), and China Postdoctoral Science Foundation (grant no. 2021M693639).
