 |
 |
- Search
AbstractObjectives. Vocal fold injection (VFI) via the cricothyroid (CT) membrane is used to treat various diseases affecting the vocal folds. The technical challenges of this technique are mainly related to the invisibility of the needle. Real-time light-guided VFI (RL-VFI) was recently developed for injection under simultaneous light guidance in the CT approach. Herein, we present the first clinical trial of RL-VFI, in which we investigated the feasibility and safety of this new technique in unilateral vocal fold paralysis (VFP).
Methods. This prospective pilot study enrolled 40 patients, who were treated with RL-VFI for unilateral VFP between September 2020 and August 2021. Adverse events were monitored during the procedure and for 4 weeks postoperatively. The Voice Handicap Index-10, the GRBAS (grade, roughness, breathiness, asthenia, and strain) scale, aerodynamic studies, and acoustic analyses were evaluated to compare the voice improvement after 4 weeks with the baseline values.
Results. The needle tip was intuitively identified by the red light. The mean procedure time was 95.6±40.6 seconds for the initial injection, while the additional injection required 79.2±70.5 seconds. The injection was performed under light guidance without additional manipulation after the needle reached the intended point. No acute or delayed adverse events were reported. Among the 40 patients, 36 completed voice analyses after 4 weeks. Subjective and objective voice parameters, including the Voice Handicap Index-10, GRBAS scale, maximum phonation time, mean expiratory airflow, fundamental frequency, jitter, shimmer, and noise-to-harmonics ratio improved significantly after RL-VFI (P<0.05), while the expiratory volume was maintained.
The vocal fold injection (VFI) technique has been utilized as an effective treatment option for unilateral vocal fold paralysis (VFP) since its introduction over a century ago [1,2]. This minimally invasive technique has been re-emphasized due to recent advances in endoscopic technology and injection materials [3,4]. Currently, VFI is widely used in the management of unilateral VFP, and its indications are broadening to a variety of vocal fold (VF) pathologies [1,5].
Various approaches have been reported for VFI, including transcutaneous (cricothyroid [CT] membrane, trans-thyroid cartilage, and thyrohyoid membrane approaches), transoral, and transnasal approaches [6,7]. Each approach has its advantages and limitations, and the choice of an approach usually depends on the surgeon’s preference and the patient’s anatomical condition [8,9]. Some laryngologists prefer the CT membrane approach because the submucosal pathway may reduce the risk of bleeding, laryngospasm, and injectate spillage. For these reasons, the CT membrane approach has accelerated the spread of office-based VFI [3,8,9].
The CT approach has been used to treat various VF diseases. In this technique, precise injection at a designated location is crucial for effective augmentation and avoiding mis-injection [10]. However, the needle tip is not visible because it moves inside the VF during the CT approach. Thus, most laryngologists discern the location of the needle tip indirectly using physical maneuvers such as CT membrane palpation and the distortion of the VF configuration [11,12]. Due to the invisibility of the needle tip, the precise localization of the needle is very difficult in the CT approach and requires a high level of experience with a steep learning curve [8,13]. Furthermore, technical proficiency in the CT approach necessitates an understanding of the anatomical orientation linking exterior landmarks with the internal laryngeal anatomy [14].
Some researchers have suggested that the invisibility of the needle tip is the primary reason for technical limitations in the CT approach [5,8]. To overcome this limitation, Cha et al. [8] conceptualized a new technique that allows injection under simultaneous light guidance, for which they coined the term “real-time light-guided VFI” (RL-VFI). In previous studies, concept models for RL-VFI were developed [8], and the technique was further verified in an ex vivo and in vivo canine model [9,15], demonstrating that the RL-VFI device might be feasible and safe by providing precise localization and visual-motor feedback.
The Ministry of Food and Drug Safety in South Korea recently approved the RL-VFI device for clinical use. To our knowledge, no reports have explored the clinical application of RL-VFI. Herein, we present the first clinical trial of RL-VFI, in which we investigate the feasibility and safety of this new technique in unilateral VFP.
This clinical study was an investigator-initiated prospective study approved by the Institutional Review Board of Seoul National University Bundang Hospital (IRB No. B-2006-621-007). The protocol was registered with the Clinical Research Information Service (CRIS; CRIS No. KCT0006375). It was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Each participant provided written informed consent before the procedure; all participants were assured anonymity.
The inclusion criteria were patients aged 18 to 85 years with unilateral VFP. The exclusion criteria were bilateral VFP, history of head and neck malignancy, history of radiotherapy in the head and neck region, history of laryngeal framework surgery, history of stenotic or obstructive lesions in the larynx or trachea, hypersensitivity, or allergic reaction to hyaluronic acid (HA) filler, and pregnancy or breastfeeding. Patients were prospectively recruited for this clinical trial from September 2020 to August 2021. We reviewed the clinical information of all the participants related to unilateral VFP. The study participants were treated with RL-VFI for unilateral VFP.
RL-VFI is a VFI technique that allows simultaneous injection under light guidance using the RL-VFI device, which provides a red light at the needle tip. The commercialized device for RL-VFI (Lightin; Solmedix Co., Seoul, Korea) comprises two components, a light source, and an injector. The light source has red light-emitting diode modules (625 nm) and emits light via a single optic fiber with controllable brightness under the maximum power of 13.2 mW. The injectors consist of a needle (1.5–inch, 23-gauge) and a connector with an optic fiber cable (Fig. 1). The insertion of the optic fiber inevitably leads to a decrease in cross-sectional area of the needle, thereby increasing injection pressure. To facilitate the injection of highly-cohesive HA or more viscous materials, the RL-VFI injector was designed with a larger needle (a 23-guage needle) than in conventional VFI (a 25-gauge needle).
All procedures were performed by a single laryngologist (WC). Patients were seated with the neck extended, and 4% lidocaine was sprayed in the nasal cavity, pharynx, and larynx. The cervical skin was anesthetized using 2% lidocaine (1:100,000 epinephrine) and sterilized with betadine. A full high-definition video laryngoscopy system comprising a video processor (EPK-i5000; Pentax Medical, Tokyo, Japan) and flexible naso-pharyngo-laryngoscope (VNL11-J10, Pentax Medical) was used to secure the procedure field and visualize the larynx. HA filler (Neuramis Deep lidocaine, 1.0 mL prefilled syringe; Medytox Inc., Cheongju, Korea) was used as the injectate.
Acute complications related to VFI were monitored, such as dyspnea, hemorrhage, severe pain, dysphagia, needle penetration, injectate leakage, and subepithelial injection. Additionally, delayed adverse events were monitored for 4 weeks postoperatively. The procedure times of RL-VFI were subsequently measured as two steps, aiming and injection. The aiming time was defined as the time from needle insertion in the skin to needle placement at the target point (just lateral to the vocal process). The injection time was defined as the time from the needle placement at the target point to injection completion. The sum of the two measurements computed the total procedure time.
The amount of injection material was determined based on the clinical findings of VF during the procedure. After sufficient injection, 10%–20% of preinjected volume was excessively injected considering the absorbed or dispersed amount after injection. If the augmentation was insufficient after the initial injection with 1 mL of commercialized volume, an additional injection was performed for proper medialization by replacing the used syringe with a new prefilled one using the same injector. The total injection volume was measured as the actual volume that was inserted into the VF, excluding the volume of the dead space (0.3 mL) formed in the connector of the device. The procedure time for the additional injection was measured using the manner described earlier.
The voice outcome of RL-VFI was evaluated prior to and 4 weeks following the procedure. Voice evaluation methods included Voice Handicap Index-10 (VHI-10), perceptual evaluation (GRBAS [grade, roughness, breathiness, asthenia, and strain] scale), acoustic analysis, and aerodynamic study. Pre- and postoperative voice data were analyzed for acoustic analysis and aerodynamic study using the Computerized Speech Lab and the Phonatory Aerodynamic System (Model 4500 and Model 6600, Pentax Medical).
Continuous variables are presented as means and standard deviations. Changes in preoperative and postoperative values were presented as the mean of each difference in preoperative and postoperative values. Change percentage was obtained from the change value compared to the baseline (preoperative) value. A paired two-tailed Student t-test was used to compare the differences in the voice outcomes pre- and postoperatively. Statistical analyses were conducted using the IBM SPSS ver. 20.0 (IBM Corp., Armonk, NY, USA); P-values <0.05 were considered statistically significant.
Out of 74 eligible patients with unilateral VFP, 9 did not meet the inclusion criteria and 25 refused to participate. Finally, 40 patients were enrolled, and they underwent RL-VFI treatment. Four of these patients did not attend the scheduled voice evaluation 4 weeks postoperatively. The remaining participants visited the hospital within 3 months, and delayed adverse events for 4 weeks were monitored. Thus, 40 patients were included for the safety evaluation and procedure time measurement, and 36 were evaluated for postoperative voice outcomes (Fig. 2).
This study included 32 men (80%) and 8 women (20%), with a mean age of 61.2±13.0 years. VFP was mostly observed on the left side (n=35, 87.5%) and was mainly caused by thoracic surgery involving the aorta (n=9, 22.5%) or the lung/esophagus (n=9, 22.5%). Participants with iatrogenic causes of VFP were mainly referred from the cardiac surgery department of our institution for adjuvant VFI therapy. Mediastinal lymph node metastases (n=14, 35.0%) and idiopathic causes (n=7, 17.5%) were other etiologies of VFP. The mean onset of VFP was 5.9±14.5 months, and most cases occurred within 6 months (Table 1).
The sequential steps of RL-VFI are demonstrated in Fig. 3. In the representative case, the left VF was paralyzed in the paramedian position and had a bowed edge (Fig. 3A). When the needle entered the skin and approached the CT membrane, the needle tip was identified by the red light emitted through the subglottic mucosa (Fig. 3B). The light on the subglottic mucosa provided information regarding the spatial orientation and intuitively helped prevent the needle from penetrating the mucosa. The needle was gently advanced into the paraglottic space and the thyroarytenoid muscle. The light could be clearly identified when it reached the target point just lateral to the vocal process (Fig. 3C). After confirming the placement of the needle at the target point under light guidance, HA was injected into the thyroarytenoid muscle (Fig. 3D). As the injectate spread, the light dispersed more through it due to the higher transmittance of the injectate than that of the adjacent tissues. The extent of the injectate was clearly indicated by light dispersion (Fig. 3E). The procedure was completed safely, and the injector was withdrawn. Thus, the left VF was effectively medialized by the injectate after the procedure (Fig. 3F).
If the initial injection did not medialize the VF sufficiently, an additional injection was administered for further augmentation, because each prefilled syringe contained 1.0 mL of HA. The additional RL-VFI was performed in the same way, using a new prefilled one with the same injector. The needle tip was clearly traceable through the injected material with dispersed light. Further augmentation of the VF could be accomplished without difficulty under light guidance (Fig. 4).
The mean volume of injectate used was 1.0±0.4 mL. Additional injections were conducted in 29 of 40 patients because further augmentation was required after the initial injection. The mean aiming time, injection time, and total procedure time during the initial injection were 22.6±18.4, 73.6±32.8, and 95.6±40.6 seconds, respectively, and those during the additional injection were 17.2±15.0, 62.0±69.3, and 79.2±70.5 seconds, respectively (Table 2). No acute complications of RL-VFI were observed in any participants. During the follow-up period, there were no procedure-related delayed adverse events or unscheduled hospital visits.
Voice outcomes were analyzed in 36 patients by comparing the preoperative and postoperative results of the voice evaluation tools (Table 3). Significant improvements were found in the VHI-10 score (33.7 vs. 24.1; change: −9.6, 28.5% of baseline; P< 0.01) and in the total GRBAS scale (6.3 vs. 4.4; change: −1.9, 30.2% of baseline; P<0.01). Aerodynamic studies demonstrated marked improvements in the maximum phonation time (change: 4.6, 109.5% of baseline; P<0.01), the mean expiratory airflow (change: −0.2, 40.0% of baseline; P=0.004), and the mean sound pressure level (74.5 vs. 77.9; change: 3.4, 4.6% of baseline; P=0.003), but not in aerodynamic efficiency (P=0.144). Acoustic analyses presented significant postoperative improvements in fundamental frequency, frequency and amplitude perturbation, as well as noise and tremor evaluations, such as fundamental frequency (P=0.049), jitter, shimmer, and the noise/harmonic ratio (all P<0.01). However, expiratory volume retained its level after the operation, and acoustic analyses of voice breaking, subharmonics, and voice irregularities did not show significant changes.
The CT approach is a minimally invasive and effective method, wherein the needle is directly inserted into the VF through the skin and CT membrane. Although its operative concept is simple, the procedure can be technically challenging [14]. The insertion point of the needle is first determined by external landmarks, and then the needle placement in the larynx is indirectly identified using the altered configuration of the VF on laryngoscopy [12]. Because the needle is invisible until it penetrates the mucosa or approaches the VF submucosa, the procedure may require several attempts even for experienced laryngologists [14]. This invisibility of the needle tip might be the crucial drawback of the CT approach [5,8].
Identifying the needle tip in the VF is the most difficult step of this approach; its location in the VF is inferred based on the distortion of the VF configuration [3], creating undesirable clinical situations. Incorrect placement of the needle tip can lead to failure to reduce the VF gap [10], and penetration of the VF mucosa may cause adverse effects such as spillage of the injectate, hematoma, and laryngeal spasm [3]. Inadvertent mis-injection in the extra-glottic space is a rare deleterious complication capable of causing pulmonary embolism [16], while mis-injection in the Reinke’s space may lead to unfavorable outcomes requiring laryngeal microsurgery [17].
Improving the precision of VFI may make the procedure reliable and predictable. Efforts have been made to increase the accuracy of the CT approach [5,11,14]. Jin et al. [14] conducted an anthropometric study of the CT approach using three-dimensionally reconstructed computed tomography. They provided anatomical information regarding the distance and angle of the path of the needle during the approach. Electromyography-guided VFI has been proposed as a good option, for which the long-term results have already been validated [18], and VFI under ultrasonographic guidance also enables real-time assessment of the position of the needle [19]. Additionally, Hoffman et al. [5] suggested an innovative idea of transillumination of the VF, which provided direct visual feedback in this approach. Although this technique was proven to be capable of localizing the needle tip, the fiberoptic cable had to be removed before delivering the injectate. Therefore, it would be difficult to apply this removal and reinsertion technique in real-world practice.
RL-VFI was conceptualized to perform simultaneous injection into the VF with the precise placement of the needle tip under light guidance [8]. The RL-VFI device allows accurate localization of the needle tip and facilitates material delivery without further manipulation of the needle after determining the target point of injection. An ex vivo study using a prototype RL-VFI device successfully demonstrated accurate localization of the needle tip [8,9]. Further research on an in vivo canine model presented successful results with various transcutaneous approaches [15], and histologic analysis of the canine larynx confirmed the presence of the injectate in the thyroarytenoid muscle, without any evidence of thermal damage to the surrounding tissues. Based on the results of preclinical studies [8,9,15], this study was designed as a pilot study—the first trial applied to humans for the purpose of investigating the feasibility and safety of RL-VFI.
This feasibility study confirmed the clear visualization and precise localization of the needle tip during RL-VFI in clinical practice. The needle tip was easily identified during the entire procedure, from penetration of the CT membrane to approaching the target point in the VF mucosa (Fig. 3). During RL-VFI, the brightness scale and scattering pattern of the light provided information on the needle depth. When the needle was positioned deeper from the surface, the light appeared blurred because it scattered during transmission. The light became more intense and focused as the needle tip approached the mucosal surface. This pattern could provide intuitive intraoperative guidance for the depth orientation and could help prevent inadvertent mucosal penetration. Moreover, navigating the needle through the injected material presented a stronger dispersion of light, which helped to estimate the extent of the injectate in real time. Although there is a limited ability to indicate the accurate orthotopic location in the injection material, the light guidance still provides information on the needle entering the “space” of injected material through light dispersion. An additional injection into any point in this space makes the material-filled sphere expand radially. Thus, this technique could facilitate precise dose control while injecting HA (Figs. 3 and 4).
To ensure the applicability of RL-VFI in real practice, the procedure times of RL-VFI were measured in two steps: aiming and injection. The aiming step, involving needle insertion in the skin and needle placement at the target point, is vital in conventional VFI via the CT approach. In the conventional CT approach, the needle tip position is approximated by the movement of the VF free edge when swinging the syringe horizontally [11,12]. This skill can only be acquired through clinical experience, and it has a steep learning curve [8,13]. This step generally requires a long time to determine the placement of the needle at the intended point because laryngologists usually depend on indirect physical maneuvers such as the distortion of the VF configuration [11,12]. RL-VFI adjunctively supports this process by providing intuitive optical signs for locating the needle tip. The mean aiming time was 22.6±18.4 seconds in this study, which is an encouraging indicator of possible improvements in the clinical efficacy of the CT approach. The injection step involves injectate delivery into the VF. It is performed simultaneously with an assessment of the augmentation degree. To determine the appropriate augmentation degree, the participants were asked to phonate during augmentation. During phonation, the light assisted in maintaining the needle tip at the intended point and helped to achieve delicate augmentation. The total procedure time was appropriate for the office-based setting, and this quantitative result supports the applicability of the procedure.
VFI for unilateral VFP improves voice outcomes, as assessed by the VHI-10, GRBAS scale, aerodynamic studies, and acoustic analyses [20-23]. For the acoustic analyses, we presented a total of 10 values consisting of two values from each measurement related to fundamental frequency, frequency and amplitude perturbation, noise and tremor evaluation, and voice breaking, subharmonics, and voice irregularity, while the maximum phonation time, mean expiratory airflow, mean sound pressure level, aerodynamic efficiency, and expiratory volume were presented as aerodynamic aspects of dysphonia [24,25]. In this study, most of the voice parameters related to unilateral VFP improved significantly after 4 weeks when compared with the baseline values. The total GRBAS scale showed significant improvements owing to improved results in grade, roughness, and breathiness. The participants were mostly referred after undergoing thoracic surgery (aorta or lung) or were receiving palliative care for metastatic disease; therefore, most participants’ underlying lung function was deteriorated, as evidenced by the expiratory volume (Table 3). The low postoperative VHI-10 and MPT values appeared to be associated with the patient group characteristics, since the participants were recruited from a tertiary referral institution that regularly supports cancer patients. Nevertheless, the observation of significant changes in VHI-10 and functional parameters such as MPT and MEA can be regarded as indicators of improvement in voice function representing effective augmentation.
VFI is known to be a rather safe procedure, but it can lead to several adverse events such as injectate spillage, needle penetration, hematoma, superficial injection into the subepithelial space, or other severe complications [3,16,17]. In our study, such complications were avoided with the aid of the light source. The light guidance was especially helpful for avoiding penetration of the mucosa or mis-injection. Furthermore, as was histologically presented in the previous in vivo study [15], clinical evidence of thermal damage (tissue shrinkage or burns) was not observed in any of our participants Moreover, there were no significant bleeding or regurgitation events related to the larger diameter of the needle (23-gauge) than the conventional needle diameter (25-gauge). Although these findings should be further validated with a larger sample, our results suggest that RL-VFI is expected to help improve the safety of VFI by advanced precision.
The clinical application of RL-VFI could improve the CT approach in various aspects. First, the blind nature of the procedure can be overcome with light guidance, leading to improved safety and efficacy. RL-VFI can ensure that the needle is guided to the appropriate location in the larynx, preventing inadvertent penetration of the VF mucosa or mis-injection at unintended locations. This characteristic of RL-VFI is also expected to improve the accessibility of various anatomical locations. Second, RL-VFI can be applied in VFI training and could shorten the learning curve by providing visual feedback [26]. This will eventually reduce the disparity in clinical results between expert and novice laryngologists because the needle location can be easily identified by intuitive guidance rather than by personal experience. Therefore, RL-VFI may improve the reproducibility of VFI via the CT approach, regardless of clinical experience. Moreover, visual feedback will also be helpful in treating patients with distorted anatomy, such as a thick neck or scar tissue, which can cause difficulties even for highly experienced laryngologists. Finally, when counseling patients, RL-VFI would help to reassure patients by enabling them to understand the procedure intuitively.
Although our clinical results of RL-VFI may be promising, our study has several limitations. First, the sample size was small. Potential adverse events may not have occurred in this study, and the functional outcomes might have been overestimated. Second, the research followed a single-arm design. Thus, the functional results need further verification. Although improvements in voice outcomes after VFI have been acknowledged in several studies, voice outcomes have large between-study heterogeneity, and the specific values may vary depending on patient factors, etiology, and injection skills [27]. After this study, we are planning a randomized controlled trial to validate the efficacy of RL-VFI based on clinical outcomes, such as the duration of adequate voice and the patient’s convenience. Third, although RL-VFI has been successfully applied in various transcutaneous approaches, including trans-thyroid cartilage and thyrohyoid, in a previous preclinical study [15], this study only validated RL-VFI with the CT approach. Lastly, we encountered a technical issue related to the device during the trial. The injector on the device was designed to connect to an optic fiber through a connector, leaving a dead space (0.3 mL) where it attaches to the syringe. In nine participants (22.5%) with an injection volume between 0.7 mL and 1.0 mL, additional filler and cost were required due to this technical issue of the dead space. Subsequent versions of this device need to address this technical issue related to dead space. In the future, a structured analysis of long-term voice outcomes and comparison of various approaches with conventional VFI are warranted to verify the potential advantages of RL-VFI.
In conclusion, RL-VFI is feasible and safe for treating patients with unilateral VFP. This technique is anticipated to improve the precision and safety of the CT approach in the treatment of unilateral VFP. This study provides a rationale for further structured clinical studies.
▪ Real-time light-guided vocal fold injection (RL-VFI) is a novel technique that allows injection under simultaneous light guidance.
▪ This study presents the first clinical application of RL-VFI along with its outcomes.
▪ RL-VFI was found to be feasible and safe for the treatment of unilateral vocal fold paralysis.
▪ This study presents the capability of RL-VFI to enhance the safety and precision of VFIs and provides a rationale for a larger comparative clinical study.
CONFLICT OF INTERESTThe corresponding author, Wonjae Cha, is the inventor of the device. Pusan National University and Pusan National University Hospital hold the patents related to the device, as granted by the Korean Intellectual Property Office (KR101699229B1), the Patent Cooperation Treaty (WO2017039193A1), and the United States Patent and Trademark Office (US15/536,419). Pusan National University and Pusan National University Hospital granted Solmedix Ltd., an exclusive license and the right to use. No other potential conflicts of interest relevant to this article were reported. NotesAUTHOR CONTRIBUTIONS Conceptualization: WC. Data curation: PGJ, RTM, GH. Formal analysis: PGJ, SHH, GH. Methodology: WJJ, WC. Project administration: WC. Visualization: PGJ, SHH, WC. Writing–original draft: GH, WC. Writing–review & editing: GH, WJJ, WC. ACKNOWLEDGMENTSThis study was supported by a National Research Foundation of Korea grant funded by the Korean government (Ministry of Science and ICT) (No. 2021R1F1A1062700).
Fig. 1.Office-based setting of real-time light-guided vocal fold injection (RL-VFI). (A) Light generator of the RL-VFI device. (B) Injector of the RLVFI device. (C) A patient is seated with the chin pointing upward (sniffing position) for the cricothyroid membrane approach. Red light is emitted from the injector. 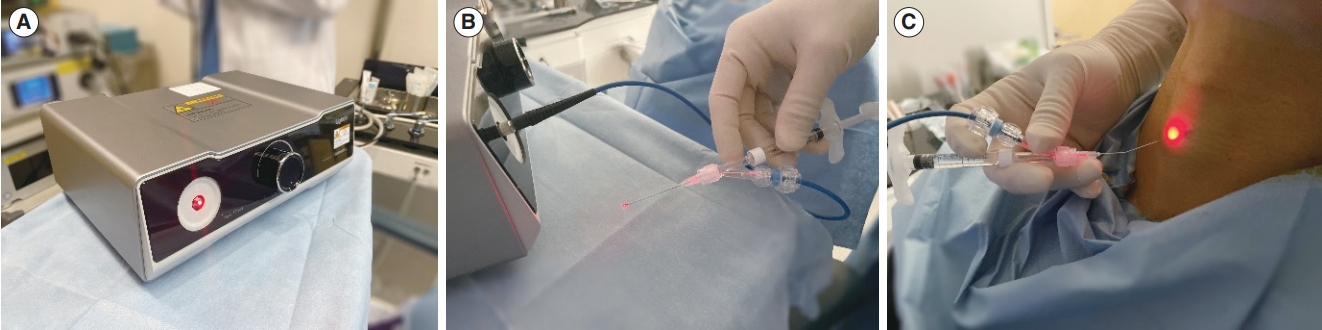
Fig. 2.Modified CONSORT (consolidated standards of reporting trials) flow diagram for this single-arm, non-randomized, preliminary study of real-time light-guided vocal fold injection. 
Fig. 3.Laryngoscopic findings during real-time light-guided vocal fold injection. (A) Preoperative findings. (B) Needle tip visualized at the subglottic mucosa on the cricothyroid membrane. (C) Needle tip placed lateral to the vocal process. (D, E) Hyaluronic acid injection into the thyroarytenoid muscle. (F) Injection completion and removal of the injector. 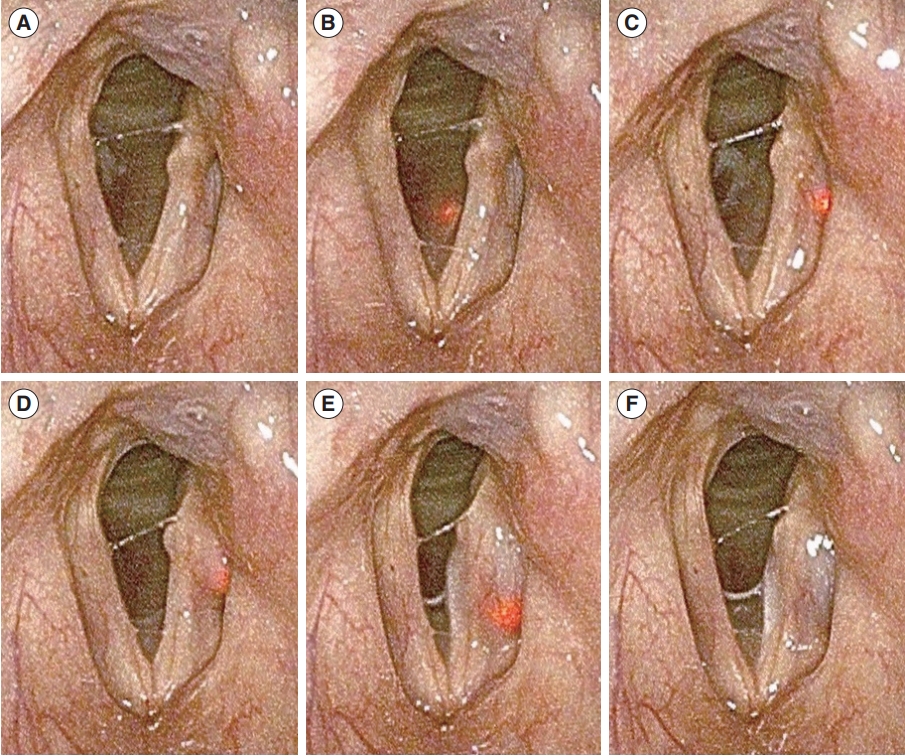
Fig. 4.Laryngoscopic findings during additional injection using the real-time light-guided vocal fold injection device. (A) After initial injection. (B) The needle is re-inserted into the previously injected material in the vocal fold. (C) Additional injection of hyaluronic acid. (D) Completion of the additional injection and removal of the injector. 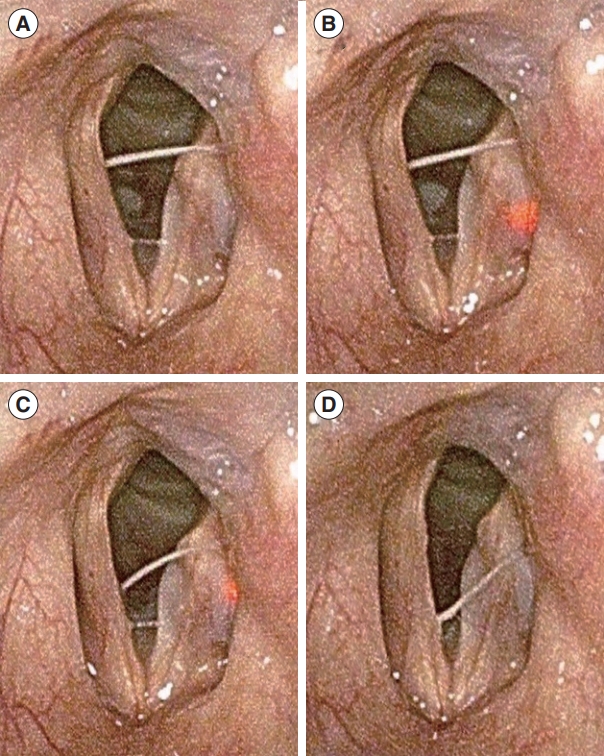
Table 1.Demographic characteristics of the study participants Table 2.Intraoperative measurements in patients treated with RL-VFI
Table 3.Voice outcomes evaluated 1 month after real-time light-guided vocal fold injection (n=36)
Values are presented as mean±standard deviation. VHI, voice handicapped index; GRBAS, grade, roughness, breathiness, asthenia, and strain; MPT, maximum phonation time; MEA, mean expiratory airflow; MSPL, mean sound pressure level; AE, aerodynamic efficiency; EV, expiratory volume; F0, fundamental frequency; STD, standard deviation of fundamental frequency; RAP, relative average perturbation; APQ, amplitude perturbation quotient; NHR, noise-to-harmonic ratio; VTI, voice turbulence index; DSH, degree of sub-harmonics; NSH, number of sub-harmonic segments. REFERENCES1. Rosow DE. Trends in utilization of vocal fold injection procedures. Otolaryngol Head Neck Surg. 2015 Nov;153(5):812-4.
2. Kwon TK, Buckmire R. Injection laryngoplasty for management of unilateral vocal fold paralysis. Curr Opin Otolaryngol Head Neck Surg. 2004 Dec;12(6):538-42.
3. Mallur PS, Rosen CA. Vocal fold injection: review of indications, techniques, and materials for augmentation. Clin Exp Otorhinolaryngol. 2010 Dec;3(4):177-82.
4. Mortensen M, Woo P. Office steroid injections of the larynx. Laryngoscope. 2006 Oct;116(10):1735-9.
5. Hoffman HT, Dailey SH, Bock JM, Thibeault SL, McCulloch TM. Transillumination for needle localization in the larynx. Laryngoscope. 2015 Oct;125(10):2341-8.
6. Seifert A. Percutaneous paraffin injection to eliminate the effects of unilateral laryngeal paralysis. Z Laryngol Rhinol Otol Ihre Grenzgeb. 1916;1916:233-5.
7. Hirano M. Transcutaneous intrafold injection for vocal fold paralysis. Trans Am Broncho-Esophago Assoc. 1985:115-7.
8. Cha W, Ro JH, Wang SG, Jang JY, Cho JK, Kim GH, et al. Development of a device for real-time light-guided vocal fold injection: a preliminary report. Laryngoscope. 2016 Apr;126(4):936-40.
9. Cha W, Ro JH, Yang SC, Choi CJ, Yang I, Kang H, et al. Real-time light-guided vocal fold injection: ex vivo feasibility study in a canine model. Laryngoscope. 2019 Apr;129(4):935-42.
10. Chheda NN, Rosen CA, Belafsky PC, Simpson CB, Postma GN. Revision laryngeal surgery for the suboptimal injection of calcium hydroxylapatite. Laryngoscope. 2008 Dec;118(12):2260-3.
11. Hirano M, Tanaka S, Tanaka Y, Hibi S. Transcutaneous intrafold injection for unilateral vocal fold paralysis: functional results. Ann Otol Rhinol Laryngol. 1990 Aug;99(8):598-604.
13. Pearson W, Hutchinson CT, Noordzij JP. Accessing the vocal folds by transcutaneous injection. Clin Anat. 2010 Apr;23(3):270-6.
14. Jin SM, Park CY, Lee JK, Ban JH, Lee SH, Lee KC. Transcutaneous injection laryngoplasty through the cricothyroid space in the sitting position: anatomical information and technique. Eur Arch Otorhinolaryngol. 2008 Mar;265(3):313-9.
15. Son HY, Kim S, Mohammad RT, Huh G, Kim H, Jeong WJ, et al. Real-time light-guided vocal fold injection: an in vivo feasibility study in a canine model. Clin Exp Otorhinolaryngol. 2021 Aug;14(3):338-46.
16. Won SJ, Woo SH. Calcium hydroxylapatite pulmonary embolism after percutaneous injection laryngoplasty. Yonsei Med J. 2017 Nov;58(6):1245-8.
17. Woo P. Hyaluronidase injection in the vocal folds for vocal hemorrhage, Reinke edema, and hyaluronic acid overinjection: a novel application in the larynx. J Voice. 2018 Jul;32(4):492-8.
18. Wang CC, Chang MH, Jiang RS, Lai HC, De Virgilio A, Wang CP, et al. Laryngeal electromyography-guided hyaluronic acid vocal fold injection for unilateral vocal fold paralysis: a prospective long-term follow-up outcome report. JAMA Otolaryngol Head Neck Surg. 2015 Mar;141(3):264-71.
19. Carrillo A, Garcia-Del-Salto L, Vaca M. Injection laryngoplasty under ultrasonographic control. Eur Arch Otorhinolaryngol. 2021 Jun;278(6):2143-6.
20. Lee SW, Kim JW, Koh YW, Shim SS, Son YI. Comparative analysis of efficiency of injection laryngoplasty technique for with or without neck treatment patients: a transcartilaginous approach versus the cricothyroid approach. Clin Exp Otorhinolaryngol. 2010 Mar;3(1):37-41.
21. Choi N, Jin H, Kim HJ, Son YI. Early injection laryngoplasty with a long-lasting material in patients with potentially recoverable unilateral vocal fold paralysis. Clin Exp Otorhinolaryngol. 2019 Nov;12(4):427-32.
22. Szkielkowska A, Miaskiewicz B, Remacle M, Krasnodebska P, Skarzynski H. Quality of the voice after injection of hyaluronic acid into the vocal fold. Med Sci Monit. 2013 Apr;19:276-82.
23. Birkent H, Sardesai M, Hu A, Merati AL. Prospective study of voice outcomes and patient tolerance of in-office percutaneous injection laryngoplasty. Laryngoscope. 2013 Jul;123(7):1759-62.
24. Hong YT, Minh PH, Hong KH. Which plosive consonant is more useful for the aerodynamic analysis of pathologic voice. Clin Exp Otorhinolaryngol. 2020 May;13(2):179-85.
25. Tanaka S, Gould WJ. Vocal efficiency and aerodynamic aspects in voice disorders. Ann Otol Rhinol Laryngol. 1985 Jan-Feb;94(1 Pt 1):29-33.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||







