 |
 |
- Search
AbstractObjectives. Multiple minimally invasive techniques for chronic rhinitis treatment focus on posterior nasal nerve ablation. We conducted a systematic review and meta-analysis to evaluate the efficacy of cryotherapy and radiofrequency ablation for alleviating symptoms in patients with allergic and nonallergic rhinitis.
Methods. We retrieved studies from PubMed, Scopus, Embase, Web of Science, and Cochrane Database up to July 2023. Data on the impact of cryotherapy and radiofrequency ablation on quality of life and symptom ratings of rhinitis were extracted and evaluated.
Results. An analysis of 12 studies involving 788 patients demonstrated significant improvements in quality of life and rhinitis-related symptoms (nasal obstruction, itching, rhinorrhea, and sneezing) in patients treated with cryotherapy or radiofrequency ablation (symptom score at 24 months and quality of life score at 3 months). However, radiofrequency ablation had a more positive effect on nasal symptoms after 3 months than cryotherapy. Nonallergic rhinitis patients responded more favorably to posterior nerve ablation than patients with allergic rhinitis. Both techniques enhanced disease-specific quality of life during the initial 3 months of treatment (cryotherapy, 84.6%; radiofrequency, 81.6%; P=0.564). After 3 months of treatment, a clinical improvement in all nasal symptoms (minimal clinically important difference in the total nasal symptom score: >1.0 points) was seen in 81.8% and 91.9% of patients who underwent cryotherapy and radiofrequency ablation, respectively (P=0.005), suggesting that radiofrequency is more likely to lead to clinical improvement.
Conclusion. Rhinitis-associated subjective symptom scores and quality of life may be improved by both cryotherapy and radiofrequency ablation. Ablation was more efficacious than cryotherapy for nasal symptoms in patients with nonallergic rhinitis. To corroborate these findings, further randomized controlled studies directly comparing these two techniques are warranted.
Chronic rhinitis, which encompasses allergic rhinitis, nonallergic rhinitis, and mixed rhinitis, is a widespread disease impacting hundreds of millions of people worldwide [1,2]. Given its high prevalence, the associated medical costs for treatment are substantial, making cost-effective treatment strategies for chronic rhinitis a critical area of research [3].
It can be challenging to distinguish among allergic rhinitis, which is triggered by immunoglobulin E-mediated inflammation in the nasal mucosa following allergen exposure, nonallergic rhinitis, and mixed rhinitis based on patients’ symptoms [1,2]. An accurate diagnosis is crucial in guiding clinicians toward the most appropriate treatment approach. The initial treatment for chronic rhinitis often involves a medical intervention; for instance, a variety of topical treatments are available, such as steroids, anticholinergics, nasal decongestants, and antihistamines [4]. However, treatment adherence is generally low among chronic rhinitis patients [5].
For patients who experience inadequate improvement in chronic rhinitis symptoms or have an aversion to long-term treatment, minimally invasive surgical options may be considered [6]. Surgical techniques such as Vidian neurectomy were initially explored; however, those procedures necessitated general anesthesia and could lead to side effects such as dry eye [4,7]. Less invasive alternative techniques for posterior nasal nerve blocking, including cryotherapy and radiofrequency ablation, have been developed and are performed under endoscopy [4,6,8-15]. One of the novel radiofrequency ablation devices has proximal and distal flexible leaflets that adjust to the patient’s anatomy, maximizing access to nerve-rich areas on the lateral wall of the nasal cavity and allowing more posterior insertion [6]. These novel techniques pose fewer risks than Vidian neurectomy, do not have serious side effects, and do not require general anesthesia [4]. Several studies have shown that these methods can improve both quality of life and symptoms in patients with chronic rhinitis [6,15]. Nonetheless, a comprehensive meta-analysis examining the impact of posterior nasal nerve ablation on chronic rhinitis has yet to be conducted, and it remains unclear whether this technique is truly clinically useful.
To the best of our knowledge, this is the first systematic review and meta-analysis comparing the effectiveness of cryotherapy and radiofrequency ablation of the posterior nasal nerve in patients with chronic rhinitis. This analysis also investigated the duration of the therapeutic effect and compared the impact of these procedures on patients with allergic and nonallergic rhinitis.
Search terms including ablation, radiofrequency, cryotherapy, minimally invasive surgery, total nasal symptom score (TNSS), Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ) score, posterior nasal nerve, nasal congestion, nasal obstruction, sneezing, itching, chronic rhinitis, nasal surgery, and nasal airway surgery were used to identify studies in PubMed, Scopus, Embase, Web of Science, and the Cochrane Database up to July 2023.
Studies involving all procedures for ablation of the posterior nasal nerve were performed in an office setting were included. However, studies not involving nasal surgery procedures such as cryotherapy or radiofrequency ablation were eliminated after two authors independently reviewed the abstracts and titles of studies published in English. If the abstract and title did not provide sufficient information for a decision, the full manuscript was thoroughly reviewed by the same two authors (YJK and SHH). Prospective or retrospective studies involving patients seeking improvement in rhinitis-related symptoms and quality of life, complaining of severe rhinitis symptoms with or without nasal obstruction, and having a high TNSS score were included. Studies including patients who underwent additional nasal procedures, such as turbinate or sinus surgery, were excluded. Studies including clinically significant anatomic obstructions that limited access to or modified the anatomy of the posterior nose or previous sinonasal surgery, active infection or open wounds in the nasal or sinus cavities were excluded. Additionally, duplicate studies were omitted. Studies lacking quantified outcomes or providing results based on data that are difficult to quantify were also excluded. In total, 12 studies were selected for this systematic review and meta-analysis. The search strategy for the included studies is summarized in Fig. 1 (Preferred Reporting Items for Systematic Reviews and Meta-Analyses [PRISMA] diagram).
Data from the included studies were independently extracted and analyzed in a standardized manner by two authors [16,17]. The quality of life score and disease-specific symptoms were evaluated before cryotherapy or radiofrequency ablation treatment and for up to 24 months posttreatment. The review protocol was registered at the Open Science Framework (https://osf.io/wf4vk/).
The cryotherapy and radiofrequency ablation treatment groups were compared with the sham treatment group, during the follow-up period and before and after treatment [4,6,8-15,18,19]. The TNSS is a validated symptom severity rating system that averages the scores for four patient-assessed symptoms: rhinorrhea, nasal congestion, nasal itching, and sneezing. Each symptom is rated on a scale of 0–3 (0=none, 1=mild, 2=moderate, or 3=severe). Based on the patient’s assessment of their symptoms during the previous 12 or 24 hours, the maximum TNSS is 12 points. The minimal clinically important difference (MCID) for the TNSS was set at 1 point. Response to treatment was considered as a reduction in total TNSS score of ≥30% (strict good response), and an at least 1-point reduction from baseline (clinical response) [20]. Another outcome of interest was the change from baseline in the RQLQ score. The validated 14-item RQLQ assesses impairments in five domains: activities, practical issues, nose symptoms, eye symptoms, and other symptoms. Each item is scored on a 7-point scale (0=no impairment, 6=maximal impairment). The MCID for the mini RQLQ is 0.4 or 0.5 points. The P-value, patient count, and grading scale data were extracted pre- and post-therapy from the included studies.
The Cochrane Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) was used to assess the caliber of nonrandomized controlled research, and the results are described in Supplementary Table 1. The ROBINS-I evaluated the quality of studies with seven assessment domains (confounding, selection of participants, classification of interventions, deviation from intended interventions, missing data, measurement of the outcomes, and selection of the reported results). Each assessment domain level and overall judgment are graded as low, moderate, serious, and critical risk of bias. The risk of bias in randomized controlled studies was assessed using the Cochrane Risk of Bias tool, as illustrated in Supplementary Table 2.
A meta-analysis of the included studies was conducted using R statistical software (version 3.4.3; R Foundation for Statistical Computing). Mean and standard deviation values were compared between the control group and cryotherapy treatment group for continuous data, with the effect size indicated by the mean difference (MD). MD values were computed in instances when the TNSS and RQLQ scale results and units were consistent across all studies. Odds ratios (ORs) were also calculated. Heterogeneity was evaluated using Cochran’s Q test and the I2 test. Publication bias was assessed using Egger’s test and a funnel plot. The Duval and Tweedie trim-and-fill method was used to estimate publication bias.
Data from 12 studies involving 788 patients were analyzed, as shown in Fig. 1. Table 1 presents a summary of the characteristics of each study [4,6,8-13,15,18,19]. Since patient information was not fully reported in all included studies, a comprehensive analysis of patient characteristics could not be performed. Table 2 displays a summary of the study bias. Due to the small number of included studies (<10), the Egger test was not conducted and a Begg funnel plot was not generated for most outcomes. However, no evidence of publication bias was found for changes in TNSS 3 months after treatment according to the Egger test and a Begg funnel plot (P=0.245).
Three months after posterior nasal nerve ablation, the good response rate (>30% reduction in the total TNSS) was 75.3% (95% confidence interval [CI], 71.08%–79.05%; I2=0.0%). The clinical response rate was 89.9% (95% CI, 83.91%–93.83%; I2=51.0%) at 3 months post-ablation (Fig. 2).
TNSS decreased relative to baseline at 1 month (MD, 3.6585; 95% CI, 3.2652–4.0517; I2=56.2%), 3 months (MD, 3.8148; 95% CI, 3.4710–4.1586; I2=62.6%), 6 months (MD, 4.2749; 95% CI, 3.6218–4.9280; I2=88.2%), and 12 months (MD, 4.3061; 95% CI, 3.7283–4.8840; I2=77%). This indicates a significant reduction in rhinitis-related symptoms after posterior nasal nerve ablation, with changes from the baseline TNSS during the follow-up period exceeding the MCID of 1 (Fig. 2).
The device type (radiofrequency ablation vs. cryotherapy devices) was not considered in the overall analysis (Table 2), which could explain the significant heterogeneity (>50%) in some results among the studies. Radiofrequency ablation devices were used in five studies of refractory rhinitis, while cryotherapy devices were employed in seven studies. Symptoms improved significantly in patients with refractory rhinitis using both device types. The effect sizes varied significantly at 3 months postoperatively between the two device types, with radiofrequency ablation devices having a more beneficial effect on rhinitis-related symptom scores. This trend persisted throughout the first 24 postoperative months, although each subgroup in this analysis included only one study; therefore, this finding should be interpreted cautiously. Moreover, the clinical response rates (≥MCID [1 point] in the total TNSS) at 3 months after cryotherapy and radiofrequency ablation were 81.8% (95% CI, 73.93%–87.72%; I2=0.0%) and 91.9% (95% CI, 87.88%–94.65%; I2=0.0%), respectively. There was a significant difference in the clinical response rates between the two device types (P=0.005).
Three months after posterior nasal nerve ablation, the clinical response rate was 82.6% (95% CI, 77.26%–86.86%; I2=0.0%). After 3 months of treatment, there was no significant difference in the clinical response rate according to whether patients underwent cryotherapy (84.6%; 95% CI, 74.83%–91.05%; I2=0.0%) or radiofrequency ablation (81.6%; 95% CI, 74.89%–86.82%; I2=0.0%; P=0.564).
As shown in Fig. 3, the RQLQ score decreased relative to baseline at 3 months after posterior nasal nerve ablation (MD, 1.5542; 95% CI, 1.4015–1.7069; I2=0.0%). There was no statistically significant difference in the RQLQ score change at 3 months posttreatment between cryotherapy (MD, 1.5131; 95% CI, 1.3169– 1.7093; I2=0.0%) and radiofrequency ablation (MD, 1.6175; 95% CI, 1.3742–1.8607; I2=0.0%; P=0.513). The Egger test was not performed, and a Begg funnel plot was not generated, due to the insufficient number of studies included (<10). Notably, there was a significant improvement in quality of life following both treatments. In particular, the RQLQ score improved from baseline during the follow-up period (MCID >0.4).
The TNSS consists of the sum of four symptom scores for rhinorrhea, nasal congestion, nasal itching, and sneezing. In some of the included studies, the changes in the individual subdomain scores were reported. For both device types, all subdomains (nasal congestion score, itching, rhinorrhea, and sneezing) improved significantly from baseline in patients with refractory rhinitis during all follow-up periods. Interestingly, the effect sizes of itching sensation during all follow-up periods and the effect sizes of rhinorrhea and sneezing at 6 months varied significantly postoperatively between the two device types, with radiofrequency ablation devices having a more beneficial effect on rhinitis-related symptom scores (Table 3).
The majority of patients in all the analyzed studies had refractory rhinitis (allergic or nonallergic). In the four studies that assessed changes in TNSS scores according to the type of rhinitis, significant TNSS changes were observed in both the allergic rhinitis and nonallergic rhinitis groups from baseline throughout the follow-up period after posterior nasal nerve ablation (Table 4).
At 3 months postoperatively, the effect sizes started to differ between the allergic rhinitis and nonallergic rhinitis groups, and this difference became statistically significant by 12 months postoperatively. Patients with refractory rhinitis experienced relief from symptoms irrespective of allergy status. However, the response was more favorable in the nonallergic rhinitis group.
Most non-randomized controlled studies were classified as having a low to moderate risk of bias because of the inherent nature of prospective studies. By contrast, a single study was rated as having a serious risk of bias as there might have been some unknown confounding factors among all participants due to its retrospective design, which finally contributed to a serious risk of bias in confounding (Supplementary Table 1). Two randomized controlled trials were classified as having some concerns because they presented deviations in the blinding of outcome assessment. Supplementary Table 2 shows the risk of bias (RoB 2.0) assessment of the included studies.
Given the low adherence to pharmacological treatment among patients with chronic rhinitis, the need for an effective surgical technique is evident [2,4]. Although studies on surgical treatments have confirmed their positive impact on chronic rhinitis, comprehensive summaries of these findings are lacking. Our study aimed to address this gap by comparing the effects of cryotherapy and radiofrequency ablation in patients with chronic rhinitis, taking into account their allergy type (allergic and nonallergic rhinitis). In our study, rhinitis-related symptom scores and quality of life were found to be improved by cryotherapy and radiofrequency ablation, with ablation being more effective for nonallergic rhinitis patients. This statistically significant result from a comprehensive meta-analysis will provide a helpful basis for clinicians when selecting this treatment for uncontrolled or refractory chronic rhinitis, leading to clinically meaningful improvements in symptoms and quality of life measures.
Treatment strategies may vary depending on the type of chronic rhinitis [2,6]. For instance, intranasal steroids are typically recommended for the treatment of allergic rhinitis, either alone or in combination with other therapies [12]. Nonallergic rhinitis, in contrast, can be managed with intranasal steroids, antihistamines, or ipratropium [21]. However, even with conservative care, disease-specific quality of life and chronic rhinitis symptoms may not improve significantly [4].
Previous surgical interventions for refractory or uncontrolled chronic rhinitis, such as Vidian neurectomy and botulinum toxin injections, have shown limitations and adverse effects [14,22-26]. In contrast, cryotherapy and radiofrequency ablation specifically target the posterior nasal nerve [4,6]. The parasympathetic nerves of the nasal mucosa, comprising both autonomic and sensory fibers of the posterior nasal nerve, play a pivotal role in regulating mucus secretion from the submucosal gland and blood flow to alleviate stromal edema, thus effectively managing the symptoms of chronic rhinitis [27]. These parasympathetic nerve fibers innervate the middle and inferior meatus of the nasal cavity [28]. Radiofrequency ablation targets the posterior lateral nasal nerve and the parasympathetic nerve of the pterygopalatine ganglion [6]. Ablation can involve a proximal or distal approach from the choana to the middle meatus [6]. Further studies focusing on the mechanism of radiofrequency ablation are needed in light of our finding that radiofrequency ablation was more effective than cryotherapy for treating nasal symptoms in patients with nonallergic rhinitis.
Cryotherapy selectively targets the postganglionic parasympathetic fibers of the Vidian nerve through the posterior nasal nerve, avoiding complications such as dry eye typically associated with Vidian neurectomy [29]. However, it is essential to note that due to anatomical variations, the symptoms of chronic rhinitis sometimes do not significantly improve following posterior nasal nerve ablation [30].
Virani et al. [4] suggested that the effectiveness of cryotherapy for both allergic and nonallergic rhinitis could be attributed to the mediating role of the posterior nasal nerve. Similarly, Ikeda et al. [31] proposed that cryotherapy might be effective in various types of chronic rhinitis due to its ability to significantly reduce gland density and inflammatory cell infiltration in the nasal mucosa.
The studies included in our meta-analysis, which analyzed 788 patients, consistently reported that both cryotherapy and radiofrequency ablation had positive effects on chronic rhinitis, with significant improvements in quality of life and rhinitis-related symptoms. Chang et al. [10] reported that the impact of cryotherapy became evident 6 months after treatment initiation and persisted for more than 9 months. An improvement was also reported 3 months after the procedure in a study on radiofrequency ablation [6]. In our study, both techniques enhanced disease-specific quality of life during the initial 3 months posttreatment. After 3 months, clinical improvement in all nasal symptoms was seen in 81.8% and 91.9% of patients who underwent cryotherapy and radiofrequency ablation, respectively. Most studies reported that mild discomfort was the only adverse effect [6]. Posttreatment experiences might include anxiety, temporary pain, headache, nasal obstruction, dry eye, or ear discomfort [8].
Our study has some limitations. First, the potential use of other oral or topical medications before or after treatment, which were not documented in the medical charts, cannot be ruled out. Second, bias related to the funding available for the devices used for radiofrequency ablation and cryotherapy is another potential issue. Further research free from industry sponsorship and conflicts of interest is necessary. Third, future research should aim to distinguish among allergic rhinitis, nonallergic rhinitis, and mixed rhinitis. While our results underscore the effectiveness of radiofrequency ablation and cryotherapy for nonallergic rhinitis, more detailed studies are required to validate this conclusion. Considering the lack of universal diagnostic criteria for chronic rhinitis, the patient group may have been heterogeneous. Fourth, since no randomized controlled studies have compared cryotherapy and radiofrequency ablation under identical conditions, more research on this topic is needed. Most of the existing studies were single-arm rather than randomized controlled studies, leading to potential bias. Fifth, given the relatively extended duration of chronic rhinitis, long-term follow-up studies of surgical treatment will be important. Lastly, there is a need for more studies that directly compare surgical treatment with other topical nasal treatments.
Cryotherapy and radiofrequency ablation can potentially improve the condition and quality of life of patients with chronic rhinitis. However, the most significant improvement in nasal symptoms tended to be observed in patients with nonallergic rhinitis who underwent radiofrequency ablation. Further randomized controlled studies with long-term follow-up that directly compare cryotherapy and radiofrequency ablation are needed to validate our findings.
▪ This study compared the effectiveness of cryotherapy and radiofrequency ablation for posterior nasal nerve in chronic rhinitis patients.
▪ The length of the therapeutic effect was considered, and the effects on patients with allergic and nonallergic rhinitis were compared.
▪ Both cryotherapy and radiofrequency ablation enhanced subjective symptom scores and quality of life associated with rhinitis.
▪ Radiofrequency ablation might be more effective than cryotherapy for nasal symptoms, especially for patients with nonallergic rhinitis.
NotesAUTHOR CONTRIBUTIONS Conceptualization: SHH. Data curation: GS, YJK. Formal analysis: SHH. Funding acquisition: SHH, YJK. Methodology: SHH, GS. Project administration: SHH. Visualization: SHH. Writing–original draft: YJK, SHH. Writing–review & editing: YJK, GS. ACKNOWLEDGMENTSThis work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (2022R1F1A1066232). This work was also supported by the Soonchunhyang University Hospital Cheonan Research Fund.
SUPPLEMENTARY MATERIALSSupplementary materials can be found online at https://doi.org/10.21053/ceo.2023.01214.
Supplementary Table 1.Risk of bias assessment of included non-randomized controlled trial methodological quality using the ROBINS-I Supplementary Table 2.Individual randomized controlled trial methodological quality Fig. 1.Flowchart illustrating the article search and selection process. ESS, endoscopic sinus surgery. 
Fig. 2.Rates of clinical response (total nasal symptom score [TNSS]≥minimal clinically important difference [MCID] of 1 point; A) and good response (>30% decrease in total TNSS; B) after 3 months. MDs in TNSS scores between baseline and 1 month (C).
MDs in TNSS scores between baseline and 3 months (D), 6 months (E), 12 months (F), and 24 months (G) after treatment (total: individuals per group). CI, confidence interval; SD, standard deviation; MD, mean difference; NA, not available.
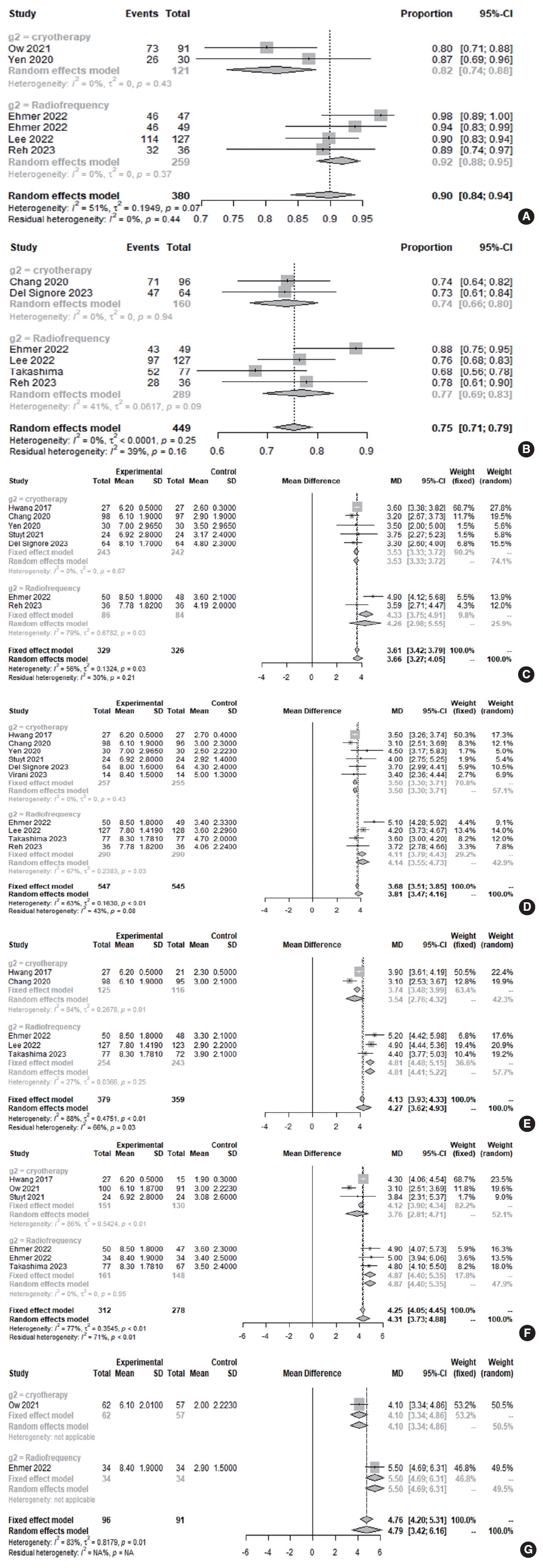
Fig. 3.Rate of clinical response (total Rhinoconjunctivitis Quality of Life Questionnaire [RQLQ] score≥minimal clinically important difference [MCID] of 0.4 or 0.5 point) at 3 months (A) and changes in RQLQ score at 3 months (B) after cryotherapy and radiofrequency ablation (total: number of participants per group). CI, confidence interval; SD, standard deviation; MD, mean difference. 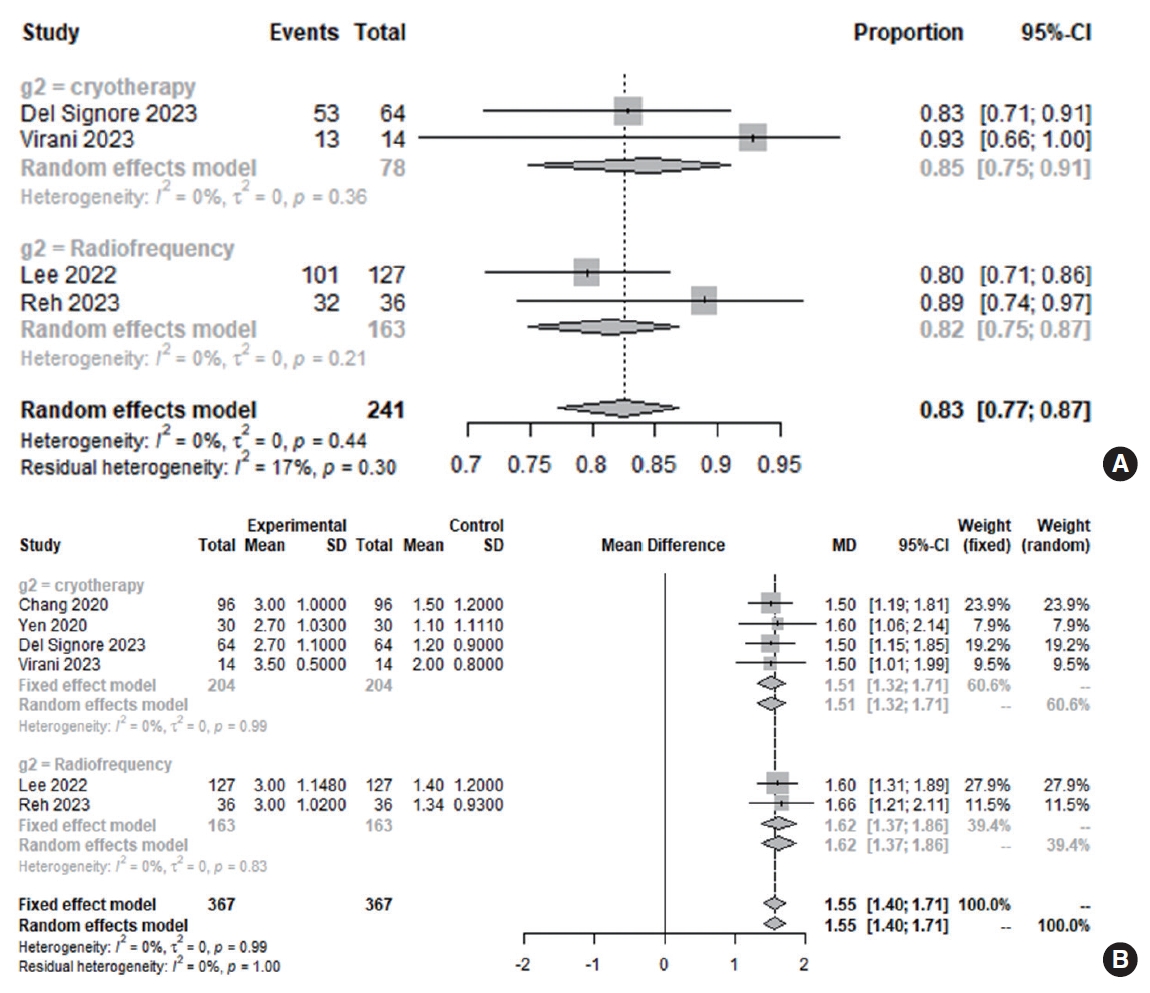
Table 1.Summary of the included studies
Table 2.Comparison of TNSS score changes from baseline to postoperative follow-up time points between the cryotherapy and radiofrequency ablation groups
Table 3.Subgroup analysis according to the subdomain of nasal symptoms in patients who underwent cryotherapy or radiofrequency ablation Table 4.Comparison of TNSS score changes from baseline to 1, 3, 6, and 12 months postoperatively between the allergic rhinitis and nonallergic rhinitis groups REFERENCES2. Settipane RA, Charnock DR. Epidemiology of rhinitis: allergic and nonallergic. Clin Allergy Immunol. 2007;19:23-34.
3. Meltzer EO, Bukstein DA. The economic impact of allergic rhinitis and current guidelines for treatment. Ann Allergy Asthma Immunol. 2011 Feb;106(2 Suppl):S12-6.
4. Virani FR, Wilson MD, Beliveau AM, Gill AS, Strong EB, Steele TO. The impact of surgical posterior nasal nerve cryoablation on symptoms and disease-specific quality of life in patients with chronic rhinitis. Ear Nose Throat J. 2023 Oct;102(10):654-60.
5. Marple BF, Fornadley JA, Patel AA, Fineman SM, Fromer L, Krouse JH, et al. Keys to successful management of patients with allergic rhinitis: focus on patient confidence, compliance, and satisfaction. Otolaryngol Head Neck Surg. 2007 Jun;136(6 Suppl):S107-24.
6. Reh DD, Lay K, Davis G, Dubin MG, Yen DM, O’Malley EM, et al. Clinical evaluation of a novel multipoint radiofrequency ablation device to treat chronic rhinitis. Laryngoscope Investig Otolaryngol. 2023 Mar;8(2):367-72.
7. Yan CH, Hwang PH. Surgical management of nonallergic rhinitis. Otolaryngol Clin North Am. 2018 Oct;51(5):945-55.
8. Del Signore AG, Greene JB, Russell JL, Yen DM, O’Malley EM, Schlosser RJ. Cryotherapy for treatment of chronic rhinitis: 3-month outcomes of a randomized, sham-controlled trial. Int Forum Allergy Rhinol. 2022 Jan;12(1):51-61.
9. Ow RA, O’Malley EM, Han JK, Lam KK, Yen DM. Cryosurgical ablation for treatment of rhinitis: two-year results of a prospective multicenter study. Laryngoscope. 2021 Sep;131(9):1952-7.
10. Chang MT, Song S, Hwang PH. Cryosurgical ablation for treatment of rhinitis: a prospective multicenter study. Laryngoscope. 2020 Aug;130(8):1877-84.
11. Yen DM, Conley DB, O’Malley EM, Byerly TA, Johnson J. Multiple site cryoablation treatment of the posterior nasal nerve for treatment of chronic rhinitis: an observational feasibility study. Allergy Rhinol (Providence). 2020 Aug;11:2152656720946996.
12. Hwang PH, Lin B, Weiss R, Atkins J, Johnson J. Cryosurgical posterior nasal tissue ablation for the treatment of rhinitis. Int Forum Allergy Rhinol. 2017 Oct;7(10):952-6.
13. Lee JT, Abbas GM, Charous DD, Cuevas PD, Goktas PD, Loftus PA, et al. Clinical and quality of life outcomes following temperaturecontrolled radiofrequency neurolysis of the posterior nasal nerve (RhinAer) for treatment of chronic rhinitis. Am J Rhinol Allergy. 2022 Nov;36(6):747-54.
14. Ehmer D, McDuffie CM, Scurry WC Jr, McIntyre JB, Mehendale NH, Willis JH, et al. Temperature-controlled radiofrequency neurolysis for the treatment of rhinitis. Am J Rhinol Allergy. 2022 Jan;36(1):149-56.
15. Stolovitzky JP, Ow RA, Silvers SL, Bikhazi NB, Johnson CD, Takashima M. Effect of radiofrequency neurolysis on the symptoms of chronic rhinitis: a randomized controlled trial. OTO Open. 2021 Sep;5(3):2473974X211041124.
16. Kim DH, Kim SW, Basurrah MA, Hwang SH. Clinical and laboratory features of various criteria of eosinophilic chronic rhinosinusitis: a systematic review and meta-analysis. Clin Exp Otorhinolaryngol. 2022 Aug;15(3):230-46.
17. Kim DH, Hwang SH. Effects of intraoperative saline-soaked pharyngeal packing on nausea, vomiting, and throat pain after nasal surgery: a systematic review and meta-analysis. J Rhinol. 2023 Mar;30(1):6-14.
18. Gerka Stuyt JA, Luk L, Keschner D, Garg R. Evaluation of in-office cryoablation of posterior nasal nerves for the treatment of rhinitis. Allergy Rhinol (Providence). 2021 Jan;12:2152656720988565.
19. Takashima M, Stolovitzky JP, Ow RA, Silvers SL, Bikhazi NB, Johnson CD. Temperature-controlled radiofrequency neurolysis for treatment of chronic rhinitis: 12-month outcomes after treatment in a randomized controlled trial. Int Forum Allergy Rhinol. 2023 Feb;13(2):107-15.
20. Ehmer D, McDuffie CM, McIntyre JB, Davis BM, Mehendale NH, Willis JH, et al. Long-term outcomes following temperature-controlled radiofrequency neurolysis for the treatment of chronic rhinitis. Allergy Rhinol (Providence). 2022 May;13:21526575221096045.
21. Klimek L, Bergmann KC, Biedermann T, Bousquet J, Hellings P, Jung K, et al. Visual analogue scales (VAS): measuring instruments for the documentation of symptoms and therapy monitoring in cases of allergic rhinitis in everyday health care: position paper of the German Society of Allergology (AeDA) and the German Society of Allergy and Clinical Immunology (DGAKI), ENT Section, in collaboration with the working group on Clinical Immunology, Allergology and Environmental Medicine of the German Society of Otorhinolaryngology, Head and Neck Surgery (DGHNOKHC). Allergo J Int. 2017 Jan;26(1):16-24.
22. Barnes ML, Vaidyanathan S, Williamson PA, Lipworth BJ. The minimal clinically important difference in allergic rhinitis. Clin Exp Allergy. 2010 Feb;40(2):242-50.
23. Juniper EF, Thompson AK, Ferrie PJ, Roberts JN. Development and validation of the mini Rhinoconjunctivitis Quality of Life Questionnaire. Clin Exp Allergy. 2000 Jan;30(1):132-40.
24. Senanayake P, Wong E, McBride K, Singh N. Efficacy of vidian neurectomy and posterior nasal neurectomy in the management of nonallergic rhinitis: a systematic review. Am J Rhinol Allergy. 2022 Nov;36(6):849-71.
25. Girdhar-Gopal H, Okurowski L, Strome M. An assessment of postganglionic cryoneurolysis for managing vasomotor rhinitis. Am J Rhinol. 1994 Jul;8(4):157-64.
27. Konno A. Historical, pathophysiological, and therapeutic aspects of vidian neurectomy. Curr Allergy Asthma Rep. 2010 Mar;10(2):105-12.
28. Ogi K, Valentine R, Suzuki M, Fujieda S, Psaltis AJ, Wormald PJ. The anatomy of the foramina and efferent nerve fibers from the pterygopalatine ganglion in posterolateral nasal wall. Laryngoscope Investig Otolaryngol. 2022 May;7(3):679-83.
29. Kikawada T. Endoscopic posterior nasal neurectomy: an alternative to vidian neurectomy. Oper Tech Otolayngol Head Neck Surg. 2007 Dec;18(4):297-301.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||







